Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
UNITED BOOK HOUSE-SET-17-EXERCISE
- Draw the Zweeterion of Glycene.
Text Solution
|
- Extablish the rate equation of a zero order reaction with single react...
Text Solution
|
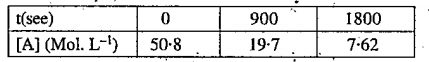
- The reaction A rarr B+C shows the result given show that it is a fist ...
Text Solution
|
- 4NH3(g)+502(g) rarr4NO(g)+6H2O(g) express instant reaction rate in ter...
Text Solution
|
- For a reaction the specific reaction rate constant at 35^@C and 65^@C ...
Text Solution
|
- What do you meanby pscudo first order reaction? Explain with proper ex...
Text Solution
|
- State with balanced Chemical equation what will happen when white phos...
Text Solution
|
- Explain why NCI3 gets hydrolysed but not NF3.
Text Solution
|
- Write name and formula of an interhalogen compound of type AX5.
Text Solution
|
- Give example of the following reactions Parkin, Clasicen Schmidt, Benz...
Text Solution
|
Text Solution
|
Text Solution
|
Text Solution
|
Text Solution
|
- What is Rochellies's salt?
Text Solution
|
Text Solution
|
Text Solution
|
- Calculate the efficiency in body centred cubic crystal.
Text Solution
|
- Which one is correct for electrolysis of CuSO4 solution using Cu elect...
Text Solution
|
- Physisorption is
Text Solution
|