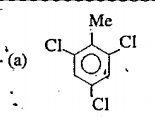
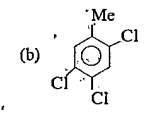
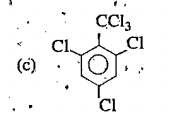
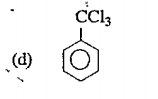
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
UNITED BOOK HOUSE-SET-17-EXERCISE
Text Solution
|
- Calculate the efficiency in body centred cubic crystal.
Text Solution
|
- Which one is correct for electrolysis of CuSO4 solution using Cu elect...
Text Solution
|
- Physisorption is
Text Solution
|
- In which acid bond present.
Text Solution
|
- H2O2 when shaken with acidified K2Cr2O7 soln in ether the ether layer ...
Text Solution
|
- Which one is an extra bitter compound ?
Text Solution
|
- When excess of CI2 passed through the solution of boiling toluene the ...
Text Solution
|
- In which reaction C-C bond is formed.
Text Solution
|
- The acid which does not respond in HVZ reaction.
Text Solution
|
- Can't be identified by Mullikan Barkar Test.
Text Solution
|
- Which base is absent in DNA
Text Solution
|
- Which one polymer contains ester linkage
Text Solution
|
- Equanil is
Text Solution
|
- Polyethylene Gloycol is used to synthesise detergent of type.
Text Solution
|
- Which cell is used in satellite or spaceship?
Text Solution
|
- What is the range of nano scale.
Text Solution
|
- Arrange as per inersasing acidic nature MnOMn2O7, MnO2
Text Solution
|
- What is used as an antioxidant in edible oil or fat preservation?
Text Solution
|
- State one difference between Drug and Medicine.
Text Solution
|