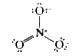
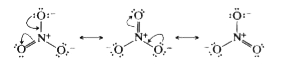
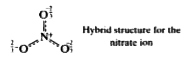Text Solution
Verified by Experts
Topper's Solved these Questions
THE BASIC BONDING AND MOLECULAR STRUCTURE
MS CHOUHAN|Exercise ADDITIONAL OBJECTIVE QUESTIONS( SINGLE CORRECT CHOICE TYPE)|22 VideosTHE BASIC BONDING AND MOLECULAR STRUCTURE
MS CHOUHAN|Exercise ADDITIONAL OBJECTIVE QUESTIONS( MULTIPLE CORRECT CHOICE TYPE)|2 VideosIUPAC NAME
MS CHOUHAN|Exercise Level 1 (Q.1 To Q.30)|1 Videos
Similar Questions
Explore conceptually related problems