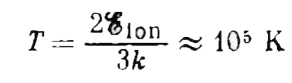Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- The ionization energy of a hydrogen atom is epsi(ion)=13.6eV. Yet the ...
Text Solution
|
- The ionization energy of H-atom is 13.6eV. Calculate the is ionizatio...
Text Solution
|
- हाइड्रोजन परमाणु की आयनन ऊर्जा है -
Text Solution
|
- How many times is the ionization energy of He^(+) ion as compared to t...
Text Solution
|
- हाइड्रोजन परमाणु की आयनन ऊर्जा है-
Text Solution
|
- हाइड्रोजन परमाणु की आयनन ऊर्जा होती है :
Text Solution
|
- हाइड्रोजन परमाणु की आयनन ऊर्जा .......... है |
Text Solution
|
- The ionization energy of a hydrogen atom is 13.6eV. The energy of the ...
Text Solution
|
- हाइड्रोजन परमाणु की आयनन ऊर्जा 13.6eV है। He^(+) तथा Li^(2+) आयनों की ...
Text Solution
|
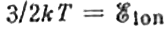 . This, however, yields too large a value for the temperature:
. This, however, yields too large a value for the temperature: