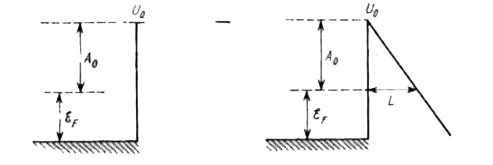
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Estimate the dimensions of a hydrogen atom in the nonexcited state, re...
Text Solution
|
- Find the kinetic energy, potential energy and total energy in first a...
Text Solution
|
- The kinetic energy of electron in the first Bohr orbit of the hydrogen...
Text Solution
|
- In hydrogen atom, energy of the first excited state is -3.4 eV . Calcu...
Text Solution
|
- Show that ground state energy of electron in hydrogen atom is equal to...
Text Solution
|
- The total energy of an electron in the first excited state of the hydr...
Text Solution
|
- Show that the energy of first excited state of He^(+) atom is equal to...
Text Solution
|
- The total energy of an electron in the first excited state of the hydr...
Text Solution
|
- If potential energy of an electron in a hydrogen atom in first excited...
Text Solution
|