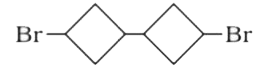
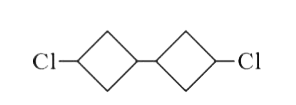
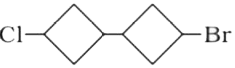
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- 1-bromo-3-chlorocyclobutane when treated with two equivalents of Na, i...
Text Solution
|
- What will be the product formed when 1-bromo-3-chlorocyclobutane react...
Text Solution
|
- 1-Bromo-3-chlorocyclobutane is treated with two equivalents of Na, in ...
Text Solution
|
- What would be the product formed when 1-bromo-3-chlorocyclobutane reac...
Text Solution
|
- 1-bromo-3-chlorocyclobutane when treated with two equivalents of Na, i...
Text Solution
|
- The product formed when 2 equivalent of metallic sodium reacts with 1-...
Text Solution
|
- When an ethereal solution of 1-bromo-3-chlorocyclobutane is treated wi...
Text Solution
|
- 1–bromo–3–chlorocyclobutane when treated with two equivalents of Na, i...
Text Solution
|
- 1–bromo–3–chlorocyclobutane when treated with two equivalents of Na, i...
Text Solution
|