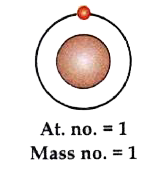Similar Questions
Explore conceptually related problems
Recommended Questions
- The diagram represents an isotope of hydrogen (H). Answer the followin...
Text Solution
|
- Hydrogen has three isotopes written as : .(1)^(1)H,.(1)^(2)H,.(1)^(3...
Text Solution
|
- हाइड्रोजन के कितने समस्थानिक रेडियोऐक्टिव होते है?
Text Solution
|
- हाइड्रोजन के कितने समस्थानिक हैं ?
Text Solution
|
- Which pair does not represent hydrogen isotopes-
Text Solution
|
- The half-life of a particular radioactive isotope is 6.5 h. If there a...
Text Solution
|
- कौनसा युग्म हाइड्रोजन समस्थानिकों को प्रदर्शित नहीं करता
Text Solution
|
- हाइड्रोजन के कितने समस्थानिक हैं ?
Text Solution
|
- समस्थानिक किसे कहते हैं ? हाइड्रोजन के कितने समस्थानिक हैं ? प्रत्येक ...
Text Solution
|