Similar Questions
Explore conceptually related problems
Recommended Questions
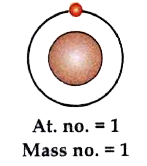
- The diagram represents an isotope of hydrogen (H). Answer the followin...
Text Solution
|
- Hydrogen has three isotopes written as : .(1)^(1)H,.(1)^(2)H,.(1)^(3...
Text Solution
|
- Which isotope of hydrogen has no neutron?
Text Solution
|
- हाइड्रोजन के कौन से समस्थायनिक के नाभिक में न्यूट्रॉन नहीं होता ?
Text Solution
|
- हाइड्रोजन के कितने समस्थानिक हैं ?
Text Solution
|
- Which isotope of hydrogen does not have neutron ?
Text Solution
|
- हाइड्रोजन के कितने समस्थानिक हैं ?
Text Solution
|
- समस्थानिक किसे कहते हैं ? हाइड्रोजन के कितने समस्थानिक हैं ? प्रत्येक ...
Text Solution
|
- The diagram represents an isotope of hydrogen (H). Answer the followin...
Text Solution
|