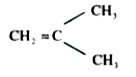
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise LECTURE SHEET ( EXERCISE-II (LEVEL-II (ADVANCED)) (Matrix Matching Type Questions)|2 VideosELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise LECTURE SHEET ( EXERCISE-II (LEVEL-II (ADVANCED)) (Integer Type Questions)|2 VideosELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise LECTURE SHEET ( EXERCISE-II (LEVEL-II (ADVANCED)) (More than One correct answer Type Questions)|6 VideosELECTRON MIGRATION EFFECTS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|10 VideosELEMENTS OF BORON FAMILY
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3|7 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ELECTRONIC EFFECTS AND REACTION INTERMEDIATES-LECTURE SHEET ( EXERCISE-II (LEVEL-II (ADVANCED)) (Linked Comprehension Type Questions)
- The delocalisation of sigma electrons with P-orbital is known as Hyper...
Text Solution
|
- The delocalisation of sigma electrons with P-orbital is known as Hyper...
Text Solution
|
- The delocalisation of sigma electrons with P-orbital is known as Hyper...
Text Solution
|
- Carbocations are species in which central carbon carries positive char...
Text Solution
|
- Carbocations are species in which central carbon carries positive char...
Text Solution
|
- Carbocations are species in which central carbon carries positive char...
Text Solution
|