A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise LECTURE SHEET ( EXERCISE-III (LEVEL-II (ADVANCED)) (Straight Objective Type Questions)|14 VideosELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise LECTURE SHEET ( EXERCISE-III (LEVEL-II (ADVANCED)) (More than correct answer Type Questions)|6 VideosELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise LECTURE SHEET ( EXERCISE-II (LEVEL-II (ADVANCED)) (Integer Type Questions)|2 VideosELECTRON MIGRATION EFFECTS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|10 VideosELEMENTS OF BORON FAMILY
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3|7 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ELECTRONIC EFFECTS AND REACTION INTERMEDIATES-LECTURE SHEET ( EXERCISE-III (LEVEL-I (MAIN)) (Straight Objective Type Questions)
- Which of the following is the strongest acid ?
Text Solution
|
- Which of the following is the weakest base ?
Text Solution
|
- The strongest base among the following
Text Solution
|
- Which of the following alcohols is the most acidic
Text Solution
|
- The most acidic compound is
Text Solution
|
- Arrange the following in the decreasing order of acidic strength
Text Solution
|
- Which of the following acids has the smallest dissociation constant ?
Text Solution
|
- Acetic acid is weaker acid than formic acid. This is explained by
Text Solution
|
- Arrange the following in the decreasing order of basic strength
Text Solution
|
- Decreasing order of acidic strength of the following compounds is
Text Solution
|
- Which is the decreasing order of acidity in, I) HCOOH " II) "CH(4)...
Text Solution
|
- Which one of the following is the strongest base in liquid phase?
Text Solution
|
- Among the following the strongest base is
Text Solution
|
- Which of the following has the most acidic hydrogen?
Text Solution
|
- Which one of the following is most acidic?
Text Solution
|
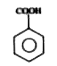 is less acidic than HCOOH & order of acidity is
is less acidic than HCOOH & order of acidity is  stabilize the Benzene.
stabilize the Benzene.