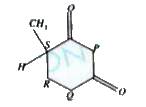
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise PRACTICE SHEET ( EXERCISE-III (LEVEL-II (ADVANCE)) (Straight Objective Type Questions)|12 VideosELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise PRACTICE SHEET ( EXERCISE-III (LEVEL-II (ADVANCE)) (More than One correct answer Type Questions)|4 VideosELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise PRACTICE SHEET ( EXERCISE-II (LEVEL-II (ADVANCE)) (Integer Type Questions)|3 VideosELECTRON MIGRATION EFFECTS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|10 VideosELEMENTS OF BORON FAMILY
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3|7 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ELECTRONIC EFFECTS AND REACTION INTERMEDIATES-PRACTICE SHEET ( EXERCISE-III (LEVEL-I (MAIN)) (Straight Objective Type Questions)
- Identify the strongest acid among the following
Text Solution
|
- It was experimentally found that formic acid is stronger acid than ace...
Text Solution
|
- The correct order of basicity of the following compounds is
Text Solution
|
- Which one of the following is the weakest base?
Text Solution
|
- Among the following compounds, the strongest acid is
Text Solution
|
- Select the correct pKa orders for the following compounds
Text Solution
|
- The correct order of acidic nature of protons in the following compoun...
Text Solution
|
- Choose the strongest base among the following:
Text Solution
|
- Among the following anions, the order of basic strength is "A)" CH(3...
Text Solution
|
- If the following compound mixed with NaOH solution. Acid base reaction...
Text Solution
|