Text Solution
Verified by Experts
Topper's Solved these Questions
STATES OF MATTER
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE - 1 (SHORT ANSWER QUESTIONS)|13 VideosSTATES OF MATTER
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE - 1 (VERY SHORT ANSWER QUESTIONS)|15 VideosSTATES OF MATTER
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL - II)(PRACTICE SHEET ADVANCED) (MORE THAN ONE CORRECT ANSWER TYPE QUESTIONS)|6 VideosSATURATED HYDROCARBONS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|10 VideosSTOICHIOMETRY
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|59 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-STATES OF MATTER-PROBLEMS
- On a ship sailing in Indian ocean where the temperture is 26.1^@C, a b...
Text Solution
|
- At STP, the volume of hydrogen is 22.72 L mol^(-1) . Calculate the vo...
Text Solution
|
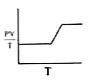
- What information is derived by the shape of the graph given for a fixe...
Text Solution
|
- Certain amount of a gas at 25^@C and 76cm Hg occupies volume of 12.0L....
Text Solution
|
- Calculate the pressure exerted by one gram of helium present in a 5 L ...
Text Solution
|
- How many grams of Chlorine can exert 1200 torr at room temperature in ...
Text Solution
|
- 0.1g of carbon dioxide occupies a volume of 320cc at certain condition...
Text Solution
|
- A synthetic mixture of nitrogen and Argon has a density of 1.4 g L^(-1...
Text Solution
|
- A container of just 10cc has 2.69 xx 10^20 gas molecules at 0^@C. What...
Text Solution
|
- One hundred Litre of a vessel contains 14g of carbondioxide at 27^@C. ...
Text Solution
|
- A 10L cylinder has helium at 8 atm and 32^@C. How many balloons of 2L ...
Text Solution
|
- An open steel vessel has an ideal gas at 27^@C. What fraction of the g...
Text Solution
|
- A vessel of irregular shape has a volume 'V'. It is first evacuated an...
Text Solution
|
- Pay load is defined as the difference between the mass of displaced ai...
Text Solution
|
- Helium diffuses 4 times faster than an unknown gas under similar condi...
Text Solution
|
- A 100 meter hollow tube of uniform thickness has two open ends X and Y...
Text Solution
|
- Elements with atomic numbers 7, 8, 9 and 10 are gases. Under similar c...
Text Solution
|
- 720cc of methane diffused through a porous membrane in 30min. Under id...
Text Solution
|
- One litre of hydrogen effused in 8min through a 8 fine aperture. What ...
Text Solution
|
- Find the ratio of rates of diffusion of hydrogen and oxygen.
Text Solution
|