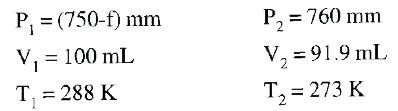
Text Solution
Verified by Experts
Topper's Solved these Questions
STATES OF MATTER
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE - 1 (SHORT ANSWER QUESTIONS)|13 VideosSTATES OF MATTER
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE - 1 (VERY SHORT ANSWER QUESTIONS)|15 VideosSTATES OF MATTER
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL - II)(PRACTICE SHEET ADVANCED) (MORE THAN ONE CORRECT ANSWER TYPE QUESTIONS)|6 VideosSATURATED HYDROCARBONS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|10 VideosSTOICHIOMETRY
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|59 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-STATES OF MATTER-PROBLEMS
- A two litre flask contains a mixture of 16g of oxygen, 7g of nitrogen ...
Text Solution
|
- 400cc of N2 at 600mm and 500cc of O2 at 300mm are quantitatively trans...
Text Solution
|
- 100cc of gas is collected over water at 15^@C and 750torr. If the gas ...
Text Solution
|
- A vessel contains helium and methane in 4:1 mole ratio at 20 bar press...
Text Solution
|
- A vessel contains 0.25 moles of Helium and 0.15 moles of neon at 298K...
Text Solution
|
- Molecules of a gas are considered as point groups. What does this sign...
Text Solution
|
- What is meant by elastic collision?
Text Solution
|
- What is the pressure exerted by 10^(23) gas molecules, each molecule o...
Text Solution
|
- Report the value of gas constant in cal K^(-1) molecule
Text Solution
|
- How many joules of translational kinetic energy is associated with 4 g...
Text Solution
|
- 7 grams of nitrogen is present at 127^@C and 16 grams of oxygen at 27^...
Text Solution
|
- 7 grams of nitrogen is present at 127^@C and 16 grams of oxygen at 27^...
Text Solution
|
- At what temperature the kinetic energy of a gas molecule is one-half o...
Text Solution
|
- Calculate the RMS, average and most probable velocity of SO2 at 27^@C.
Text Solution
|
- The average velocity of nitrogen at 27^@C is 0.3ms^(-1). At what tempe...
Text Solution
|
- At what temperature hydrogen has the same RMS velocity as that of oxy...
Text Solution
|
- Density of a gas at one atm pressure is 1.43xx10^(-2)g c c^(-1). Calcu...
Text Solution
|
- Under similar conditions, what is the ratio of RMS velocity of ozone m...
Text Solution
|
- A gas X,Y, at 35^@C has RMS speed 12ms^(-1). On heating the gas twice ...
Text Solution
|
- The ratio of rates of diffusion of two gases x and y under similar con...
Text Solution
|