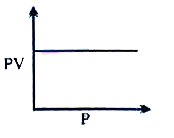
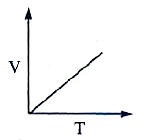
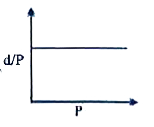
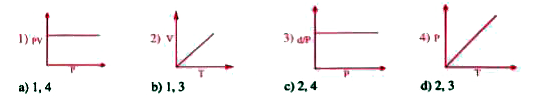A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -2|140 VideosSTATES OF MATTER
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -3|19 VideosSTATES OF MATTER
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE -3 (VERY SHORT ANSWER QUESTIONS)|18 VideosSATURATED HYDROCARBONS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|10 VideosSTOICHIOMETRY
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|59 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-STATES OF MATTER-OBJECTIVE EXERCISE -1
- 1 mole of any gas a) Occupies 22.4 lit at STP b) Contains 3.05 xx ...
Text Solution
|
- From the graph the correct order of temperatures is
Text Solution
|
- Which of the following indicates the isotherms?
Text Solution
|
- Which of the following indicates Charles' law mathematically (when n, ...
Text Solution
|
- At absolute zero which of the following statements about an ideal gas ...
Text Solution
|
- The density of CO2 gas at 27^@C and 1 atm pressure is (gram/lit)
Text Solution
|
- Which of the following indicates correct R (Gas constant) values in di...
Text Solution
|
- When the absolute temperature of a gas is doubled then the correct sta...
Text Solution
|
- Match the following The correct match is
Text Solution
|
Text Solution
|
- Match the following columns
Text Solution
|
- Which among the following indicates change in the chemical composition...
Text Solution
|
- For an ideal gas, number of moles per lit in terms of pressure (P), ga...
Text Solution
|
- From the graph the correct order of temperature is
Text Solution
|
- For an ideal gas the graph between PV/RT and T is
Text Solution
|
- One mole of argon will have least density at
Text Solution
|
- What are the conditions under which the relation between 'V' and 'n' a...
Text Solution
|
- Which of the following shows ideal gas behaviour
Text Solution
|
- When universal gas constant (R) is divided by Avogadro number(N), then...
Text Solution
|
- From the graph the order of pressure of a gas is
Text Solution
|