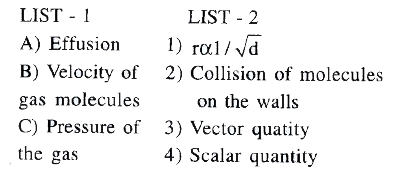A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -2|140 VideosSTATES OF MATTER
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -3|19 VideosSTATES OF MATTER
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE -3 (VERY SHORT ANSWER QUESTIONS)|18 VideosSATURATED HYDROCARBONS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|10 VideosSTOICHIOMETRY
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|59 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-STATES OF MATTER-OBJECTIVE EXERCISE -1
- The rate of diffusion of a gas at constant temperature and pressure is...
Text Solution
|
- Among the following, the applications of Graham's law are a) In Mars...
Text Solution
|
- Match the following.
Text Solution
|
- Pick out the pair of gases with the same rate of diffusion
Text Solution
|
- For the diffusion of a gas at pressure P, the rate of diffusion is exp...
Text Solution
|
- A gas diffuses four times as quickly as oxygen. The molecular weight o...
Text Solution
|
- The decreasing order of rates of diffusion of H2 , N2 , O2, and CO2, ...
Text Solution
|
- 12L of SO2 gas diffused through a porous membrane in 20 minutes. Under...
Text Solution
|
- Dalton's law of partial pressures is applicable to
Text Solution
|
- Which of the following indicates the mathematical expression for Dalio...
Text Solution
|
- Combination that obeys Dalton's law A = CO " " B = CI2 " " C ...
Text Solution
|
- A vessel contains a mixture of equal weights of oxygen and SO2 at a pr...
Text Solution
|
- A vessel contains methane and oxygen in the mass ratio 2:1. The fracti...
Text Solution
|
- The measurement of the pressure of dry gas collected over water is bas...
Text Solution
|
- Aqueous tension of water depends on
Text Solution
|
- O2 and He are taken in equal weights in a vessel. The pressure exerted...
Text Solution
|
- Equal volumes of two jars contain HCI, NH3 gases respectively at const...
Text Solution
|
- The wrong statement in the case of an ideal gas is
Text Solution
|
- An ideal gas cannot be liquified because
Text Solution
|
- The most ideal gas among real gases is
Text Solution
|