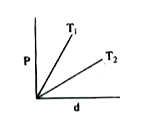
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -3|19 VideosSTATES OF MATTER
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -1|97 VideosSATURATED HYDROCARBONS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|10 VideosSTOICHIOMETRY
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|59 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-STATES OF MATTER-OBJECTIVE EXERCISE -2
- 0.2 g of a gas 'x' occupy a volume of 440 ml . If 0.1 g of CO2 gas oc...
Text Solution
|
- N2 gas is present in one litre flask at a pressure of 7.6 xx 10^(-10)m...
Text Solution
|
- Diagram shows a graph between pressure and density for an ideal gas at...
Text Solution
|
- A sample of gas has a volume of 0.2 lit measured at 1 atm pressure an...
Text Solution
|
- If the weight of 5.6 lit of a gas at NTP is 11g, the gas may be (at.wt...
Text Solution
|
- The volume of 10 moles of an ideal gas at 27^@C and latm pressure is 1...
Text Solution
|
- At 27^@C, one mole of an ideal gas exerted a pressure of 0.821 atmosph...
Text Solution
|
- A bulb of unknown volume 'V' Contains an ideal gas at 2 atm pressure. ...
Text Solution
|
- 2gm of hydrogen is present in a closed vessel at S.T.P. If the same qu...
Text Solution
|
- An open vessel at 27^(@) C is heated until 2/5 th of the air in it has...
Text Solution
|
- An open bulb containing air at 19^(@)C was cooled to a certain temper...
Text Solution
|
- A five litre flask contains 3.5gm of N2 3g of H2 and 8g of O2 at 27^@C...
Text Solution
|
- If 10 gm of a gas at atmospheric pressure is cooled from 273°C to 0°C ...
Text Solution
|
- The molecular weights of two ideal gases A and B are respectively 100 ...
Text Solution
|
- A gas occupies a volume of 300 cc at 27^@C and 620 mm pressure. The vo...
Text Solution
|
- Two samples of gases 'a' and 'b' are at the same temperature. The mole...
Text Solution
|
- A bulb of unknown volume 'V' Contains an ideal gas at 2 atm pressure. ...
Text Solution
|
- Rate of diffusion of gases is not influenced by
Text Solution
|
- Under the same conditions the rates of diffusion of two gases are in t...
Text Solution
|
- The rate of diffusion of methane at given temperature is twice that of...
Text Solution
|