Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-STUDY OF COMPOUNDS OF NITROGEN- AMMONIA -Questions from Preivous ICSE Board Papers
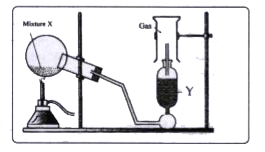
- The diagram shows an experimental set up for the laboratory preparatio...
Text Solution
|
- The diagram shows an experimental set up for the laboratory preparatio...
Text Solution
|
- The diagram shows an experimental set up for the laboratory preparatio...
Text Solution
|
- The diagram shows an experimental set up for the laboratory preparatio...
Text Solution
|
- Write balanced chemical equations for each of the following: When ex...
Text Solution
|
- Name the gas in the following: The gas produced when excess ammonia re...
Text Solution
|
- Some word/words are missing in the following statement. You are requir...
Text Solution
|
- Give balanced equation for the following reaction : Ammonia and Oxygen...
Text Solution
|
- The following questions are based on the preparation of ammonia gas in...
Text Solution
|
- The following questions are based on the preparation of ammonia gas in...
Text Solution
|
- The following questions are based on the preparation of ammonia gas in...
Text Solution
|
- The following questions are based on the preparation of ammonia gas in...
Text Solution
|
- State one appropriate observation for the following: Excess of chlorin...
Text Solution
|
- Choose the most appropriate answer from the following options : Nitr...
Text Solution
|
- Give balanced equation for the following: Reduction of hot Copper(II...
Text Solution
|
- Copy and complete the following table relating to important industrial...
Text Solution
|
- Identify : An alkaline gas which produces dense white fumes when react...
Text Solution
|
- Fill in the blank from the choices given within brackets : Ammonia g...
Text Solution
|
- Write balanced equation for the following: Action of warm water on m...
Text Solution
|
- State your observation in the following case: When calcium hydroxide i...
Text Solution
|