Text Solution
Verified by Experts
Topper's Solved these Questions
INTERNAL ENERGY
ICSE|Exercise SELECTED PROBLEMS (FROM FIRST LAW OF THERMODYNAMICS )|6 VideosINTERNAL ENERGY
ICSE|Exercise SELECTED PROBLEMS (FROM ISOTHERMAL AND ADIABATIC PROCESSES )|10 VideosINTERNAL ENERGY
ICSE|Exercise SHORT ANSWER QUESTIONS |30 VideosGRAVITATION
ICSE|Exercise FROM THE HUBBLE TELESCOP|2 VideosMOTION IN FLUIDS
ICSE|Exercise SELECTED PROBLEMS (FROM POISEUILLE.S FORMULA) |19 Videos
Similar Questions
Explore conceptually related problems
ICSE-INTERNAL ENERGY -VERY SHORT ANSWER QUESTIONS
- "A match stick can be lighted by rubbing it against a rough surface". ...
Text Solution
|
- In what way does the temperature of water at the bottom of a waterfall...
Text Solution
|
- "It is not advisable to put on wet clothes". Why?
Text Solution
|
- Is it possible that the specific heat capacity of a gas is zero as wel...
Text Solution
|
- Is it possible to heat a body without causing any rise in temperature ...
Text Solution
|
- Mention two factors on which the degrees of freedom of a gas depends.
Text Solution
|
- Which one of the following substances has highest specific heat capac...
Text Solution
|
- Weight of a body of mass m decreases by 1% when it is raised to height...
Text Solution
|
- What is the change in internal energy of a system undergoing a cyclic ...
Text Solution
|
- The relationship between enthalpy and internal energy change is
Text Solution
|
- Is it possible in increase the temperature of a gas without adding hea...
Text Solution
|
- A planet in a distant solar system is 10 times more massive than the e...
Text Solution
|
- The second law of thermodynamics is a fundamental law of science. In t...
Text Solution
|
- Milk is poured into a cup of tea and is mixed with a spoon. Is this an...
Text Solution
|
- What is the effect of pressure on the fusion and boiling point of wate...
Text Solution
|
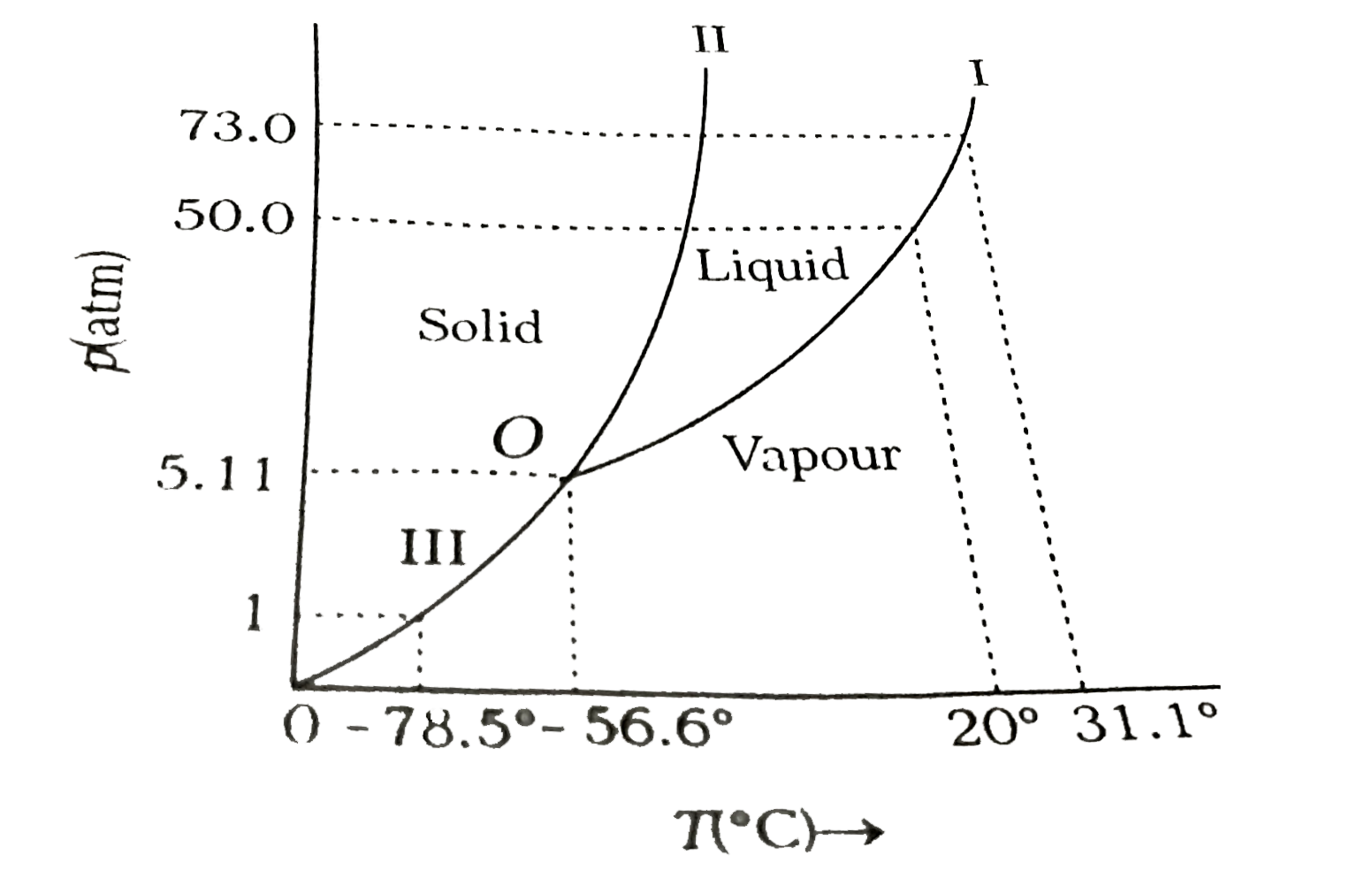
- Answer the following questions based on the p-T phase diagram of carbo...
Text Solution
|
- The energy that is transferred from a body at a higher temperature to ...
Text Solution
|