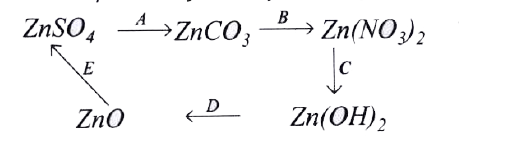Text Solution
Verified by Experts
Topper's Solved these Questions
STUDY OF ACIDS, BASES AND SALTS
ICSE|Exercise QUESTIONS FOR PRACTICE ON EXAMINATION PATTERN (SECTION I)|61 VideosSTUDY OF ACIDS, BASES AND SALTS
ICSE|Exercise QUESTIONS FOR PRACTICE ON EXAMINATION PATTERN (SECTION II)|13 VideosSTUDY OF ACIDS, BASES AND SALTS
ICSE|Exercise IMPORTANT ASSIGNMENTS|6 VideosSPECIMEN QUESTION PAPER (CHEMISTRY)
ICSE|Exercise Questions|40 VideosSTUDY OF COMPOUNDS - AMMONIA
ICSE|Exercise UNIT TEST PAPER 7B - AMMONIA |22 Videos
Similar Questions
Explore conceptually related problems
ICSE-STUDY OF ACIDS, BASES AND SALTS-ILLUSTRATIVE ASSIGNMENTS
- Some methods used for the laboratory preparation of salts are (a) me...
Text Solution
|
- Choosing only substances from the list given in the box below, write e...
Text Solution
|
- Write chemical equations for the laboratory preparation of the followi...
Text Solution
|
- Write chemical equations for the laboratory preparation of the followi...
Text Solution
|
- Write chemical equations for the laboratory preparation of the followi...
Text Solution
|
- Write chemical equations for the laboratory preparation of the followi...
Text Solution
|
- From the formulae listed in the box, choose one in each case correspon...
Text Solution
|
- Write equations for the laboratory preparation of: sodium sulphate u...
Text Solution
|
- Write equations for the laboratory preparation of: lead sulphate usi...
Text Solution
|
- Write balanced chemical equations for the preparation of the following...
Text Solution
|
- Write balanced chemical equations for the preparation of the following...
Text Solution
|
- Write balanced chemical equations for the preparation of the following...
Text Solution
|
- Write balanced chemical equations for the preparation of the following...
Text Solution
|
- Write balanced equations for the following reactions : Lead sulphate...
Text Solution
|
- Write balanced equations for the following reactions: Copper sulphat...
Text Solution
|
- Write balanced equations for the following reactions: Lead chloride ...
Text Solution
|
- Give the equations for the following conversions A to E.
Text Solution
|
- Write a balanced chemical equation for the preparation of each of the ...
Text Solution
|
- Write a balanced chemical equation for the preparation of each of the ...
Text Solution
|