Topper's Solved these Questions
CHEMICAL BONDING
ICSE|Exercise WORKSHEET-3(Tick the correct answer) |5 VideosCHEMICAL BONDING
ICSE|Exercise Additional Questions for Practice|32 VideosCHEMICAL BONDING
ICSE|Exercise WORKSHEET-3(Give one word for the following) |10 VideosANALYTICAL CHEMISTRY-USE OF AMMONIUM & SODIUM HYDROXIDE
ICSE|Exercise Additional Questions|6 VideosCHEMISTRY 2011
ICSE|Exercise SECTION-II|48 Videos
Similar Questions
Explore conceptually related problems
ICSE-CHEMICAL BONDING -WORKSHEET-3
- Complete the following electron dot diagrams : underset("Ammonia")(H...
Text Solution
|
- Complete the following electron dot diagrams : underset("Water")(H:u...
Text Solution
|
- Complete the following electron dot diagrams : underset("Water")(H:u...
Text Solution
|
- Explain : Cation has smaller size than neutral atom. ……………………………...
Text Solution
|
- Explain : Anion has larger size than neutral atom. ……………………………………...
Text Solution
|
- Explain: Electrolysis can distinguish between covalent and ionic com...
Text Solution
|
- Explain: Cation has a smaller size than parent atom. ………………………………...
Text Solution
|
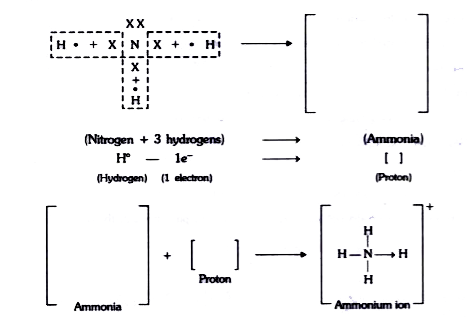
- Complete the following flow chart related to the structure of ammoniu...
Text Solution
|