Topper's Solved these Questions
CHEMICAL BONDING
ICSE|Exercise Questions from Previous ICSE Board Papers (2005)|7 VideosCHEMICAL BONDING
ICSE|Exercise Questions from Previous ICSE Board Papers (2006)|5 VideosCHEMICAL BONDING
ICSE|Exercise WORKSHEET-3(Tick the correct answer) |5 VideosANALYTICAL CHEMISTRY-USE OF AMMONIUM & SODIUM HYDROXIDE
ICSE|Exercise Additional Questions|6 VideosCHEMISTRY 2011
ICSE|Exercise SECTION-II|48 Videos
Similar Questions
Explore conceptually related problems
ICSE-CHEMICAL BONDING -Additional Questions for Practice
- Draw the structure of ammonia molecule.
Text Solution
|
- Explain the following: Ionic compounds conduct electricity.
Text Solution
|
- Explain the following: lonic compounds have high melting point and ...
Text Solution
|
- Explain the following: Ionic compounds dissolve in water whereas c...
Text Solution
|
- Explain the following: lonic compounds are usually hard crystals.
Text Solution
|
- Predict the type of bonding in the following molecules: Oxygen
Text Solution
|
- Predict the type of bonding in the following molecules: calcium ox...
Text Solution
|
- Predict the type of bonding in the following molecules: water
Text Solution
|
- Predict the type of bonding in the following molecules: methane
Text Solution
|
- Predict the type of bonding in the following molecules: ammonium i...
Text Solution
|
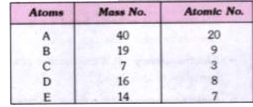
- Five atoms are labelled from A to E Which one of these atoms : ...
Text Solution
|
- Five atoms are labelled from A to E Write down the formula of t...
Text Solution
|
- Five atoms are labelled from A to E Predict which are : (i) m...
Text Solution
|
- Match the atomic number 4, 14, 8, 15 and 19 with each of the followin...
Text Solution
|
- Match the atomic number 4, 14, 8, 15 and 19 with of the following: ...
Text Solution
|
- Match the atomic number 4, 14, 8, 15 and 19 with of the following: ...
Text Solution
|
- Match the atomic number 4, 14, 8, 15 and 19 with of the following: ...
Text Solution
|
- Elements X, Y and Z have atomic numbers 6, 9 and 12 respectively. Whi...
Text Solution
|
- Elements X, Y and Z have atomic numbers 6, 9 and 12 respectively. Whi...
Text Solution
|
- Elements X, Y and Z have atomic numbers 6, 9 and 12 respectively. Whi...
Text Solution
|