Topper's Solved these Questions
STUDY OF ACIDS, BASES AND SALTS
ICSE|Exercise FILL IN THE BLANKS|24 VideosSTUDY OF ACIDS, BASES AND SALTS
ICSE|Exercise MULTIPLE CHOICE QUESTIONS|46 VideosSTUDY OF ACIDS, BASES AND SALTS
ICSE|Exercise Additional Questions for Practice|41 VideosSPECIMEN QUESTION PAPER (CHEMISTRY)
ICSE|Exercise Questions|40 VideosSTUDY OF COMPOUNDS - AMMONIA
ICSE|Exercise UNIT TEST PAPER 7B - AMMONIA |22 Videos
Similar Questions
Explore conceptually related problems
ICSE-STUDY OF ACIDS, BASES AND SALTS-Questions from Previous ICSE Board Papers
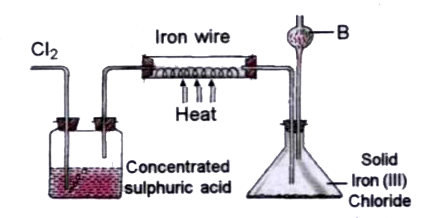
- The diagram given below is to prepare Iron (III) chloride in the labor...
Text Solution
|
- The diagram given below is to prepare Iron (III) chloride in the labor...
Text Solution
|
- The diagram given below is to prepare Iron (III) chloride in the labor...
Text Solution
|
- Equation(s) for the reaction(s) to prepare lead sulphate from lead car...
Text Solution
|
- Define neutralisation.
Text Solution
|
- Select from the below given (a) and (b) one substances in each case wh...
Text Solution
|
- Select from the below given (a) and (b) one substances in each case wh...
Text Solution
|
- An example of a complex salt is :
Text Solution
|
- Give the equation for the preparation of each of the following salts ...
Text Solution
|
- Give the equation for the preparation of each of the following salts ...
Text Solution
|
- Give the equation for the preparation of each of the following salts ...
Text Solution
|
- Give the equation for the preparation of each of the following salts ...
Text Solution
|
- Write the balanced chemical equation for each of the following reactio...
Text Solution
|
- Name the method used for preparation of the following salts from the l...
Text Solution
|
- State one observation for the following : A zinc granule is added to c...
Text Solution
|
- Match the following:
Text Solution
|
- From the list given below, select the word(s) required to correctly co...
Text Solution
|
- State one appropriate observation for each of the following: Copper...
Text Solution
|
- Give suitable chemical terms for the following : A salt formed by in...
Text Solution
|
- Give suitable chemical terms for the following : A definite number o...
Text Solution
|