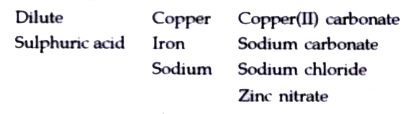Topper's Solved these Questions
STUDY OF ACIDS, BASES AND SALTS
ICSE|Exercise FILL IN THE BLANKS|24 VideosSTUDY OF ACIDS, BASES AND SALTS
ICSE|Exercise MULTIPLE CHOICE QUESTIONS|46 VideosSTUDY OF ACIDS, BASES AND SALTS
ICSE|Exercise Additional Questions for Practice|41 VideosSPECIMEN QUESTION PAPER (CHEMISTRY)
ICSE|Exercise Questions|40 VideosSTUDY OF COMPOUNDS - AMMONIA
ICSE|Exercise UNIT TEST PAPER 7B - AMMONIA |22 Videos
Similar Questions
Explore conceptually related problems
ICSE-STUDY OF ACIDS, BASES AND SALTS-Questions from Previous ICSE Board Papers
- Choosing the substances from the list given below, write balanced chem...
Text Solution
|
- Choosing the substances from the list given below, write balanced chem...
Text Solution
|
- Choosing the substances from the list given below, write balanced chem...
Text Solution
|
- Choosing the substances from the list given below, write balanced chem...
Text Solution
|
- Fill in the blank from the choices given within bracket: The basicit...
Text Solution
|
- Dilute sulphuric acid and dilute hydrochloric acid (using barium chlor...
Text Solution
|
- Give balanced chemical equations to prepare the following salts: Le...
Text Solution
|
- Give balanced chemical equations to prepare the following salts: S...
Text Solution
|
- Give balanced chemical equations to prepare the following salts: Co...
Text Solution
|
- Identify the acid which matches the following description : The acid...
Text Solution
|
- Identify the acid which matches the following description : The ele...
Text Solution
|
- Identify the acid which matches the following description : Give bal...
Text Solution
|
- Choose the most appropriate answer from the following list of oxides w...
Text Solution
|
- Choose the most appropriate answer from the following list of oxides w...
Text Solution
|
- Choose the most appropriate answer from the following list of oxides w...
Text Solution
|
- Choose the most appropriate answer from the following list of oxides w...
Text Solution
|
- From the list of the following salts choose the salt, that most approp...
Text Solution
|
- From the list of the following salts choose the salt, that most approp...
Text Solution
|
- From the list of the following salts choose the salt, that most approp...
Text Solution
|
- Fill in the blank with the choices given in brackets. Higher the pH ...
Text Solution
|