Topper's Solved these Questions
ELECTROLYSIS
ICSE|Exercise ADDITIONAL QUESTIONS FOR PRACTICE |49 VideosELECTROLYSIS
ICSE|Exercise QUESTIONS FROM PREVIOUS ICSE BOARD PAPERS|96 VideosELECTROLYSIS
ICSE|Exercise WORK SHEET -3 (Answer the following questions : )|4 VideosCHEMISTRY-2015
ICSE|Exercise SECTION-II (40 Marks) Attempt any four questions from this Section.|49 VideosMETALLURGY
ICSE|Exercise UNIT TEST PAPER 6 - Metallurgy (Select the correct answer from the list A,B,C & given in each statement.) |5 Videos
Similar Questions
Explore conceptually related problems
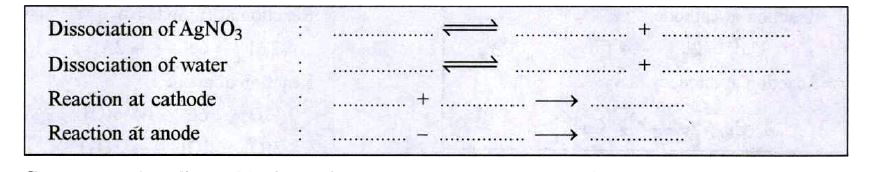
ICSE-ELECTROLYSIS-WORK SHEET -4 (complete the following : )
- Electrolysis of Silver nitrate using silver electrodes. Complete the t...
Text Solution
|
- Complete the reactions for electrolysis of concentrated sodium chlorid...
Text Solution
|
- Molten nickel sulphate using nickel electrode dissociation of NiSO4
Text Solution
|
- Dilute sodium chloride using gaphite electrodes.
Text Solution
|
- Aqueous sodium hydroxide using graphite electrode
Text Solution
|