Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-METALLURGY-QUESTIONS FROM PREVIOUS ICSE BOARD PAPERS
- Find the odd one out and explain your choice (note:valency is not a cr...
Text Solution
|
- Correct the following statements.For Example : "Chlorine is a bleachin...
Text Solution
|
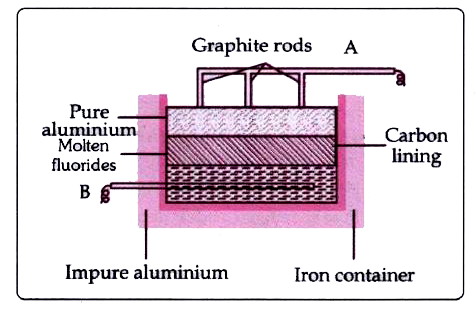
- The sketch below illustrates the refining of aluminium by Hoope's proc...
Text Solution
|
- The sketch below illustrates the refining of aluminium by Hoope's proc...
Text Solution
|
- The sketch below illustrates the refining of aluminium by Hoope's proc...
Text Solution
|
- State the property of the metal being utilised in the following:
Text Solution
|
- Which one of the following is not true of metals
Text Solution
|
- Give the equations for the following conversions A to E
Text Solution
|
- Name the main constituent metal in the following alloy : Duralumin
Text Solution
|
- Name the main constituent metal in the following alloys : Brass
Text Solution
|
- Name the main constituent metal in the following alloys : Stainless...
Text Solution
|
- Choose from the following list of substances, as to what matches the d...
Text Solution
|
- What would you observe in each of the following cases? When carbon m...
Text Solution
|
- This metal is a liquid at room temperature.
Text Solution
|
- Name a metal which is found abundantly in the earth's crust.
Text Solution
|
- What is the difference between calcination and roasting?
Text Solution
|
- Name the process used for the enrichment of sulphide ore.
Text Solution
|
- Write the chemical formulae of one main ore of iron and aluminium .
Text Solution
|
- Write the constituents of electrolyte for the extraction of aluminium.
Text Solution
|
- Which of the following metallic oxides cannot be reduced by normal red...
Text Solution
|