Topper's Solved these Questions
PRACTICAL CHEMISTRY
ICSE|Exercise Questions from Previous ICSE Board Papers (2007) |1 VideosPRACTICAL CHEMISTRY
ICSE|Exercise Questions from Previous ICSE Board Papers (2008) |5 VideosPRACTICAL CHEMISTRY
ICSE|Exercise WORKSHEET (Tick the correct options) |5 VideosPERIODIC TABLE (PERIODIC PROPERTIES AND VARIATION OF PROPERTIES)
ICSE|Exercise UNIT TEST PAPER 1-Periodic Table|22 VideosQUESTION PAPER 2022 TERM 1
ICSE|Exercise MULTIPLE CHOICE QUESTIONS|40 Videos
Similar Questions
Explore conceptually related problems
ICSE-PRACTICAL CHEMISTRY -Additional Questions for Practice
- How will you distinguish between the following pairs of gases: Sul...
Text Solution
|
- How will you distinguish between the following pairs of gases: Hydr...
Text Solution
|
- How will you distinguish between the following pairs of gases: Hydr...
Text Solution
|
- How will you distinguish between the following pairs of gases: Hydr...
Text Solution
|
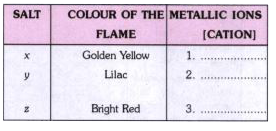
- The metallic ions (the cations) can be identify by performing flame t...
Text Solution
|
- Match the following:
Text Solution
|
- Describe all that you would observe when the following compounds are ...
Text Solution
|
- Describe all that you would observe when the following compounds are ...
Text Solution
|
- Describe all that you would observe when the following compounds are ...
Text Solution
|
- Describe all that you would observe when the following compounds are ...
Text Solution
|
- Describe all that you would observe when the following compounds are ...
Text Solution
|
- Describe all that you would observe when the following compounds are ...
Text Solution
|
- Describe all that you would observe when the following compounds are ...
Text Solution
|
- What happens when ammonium hydroxide and sodium hydroxide solutions a...
Text Solution
|
- What happens when ammonium hydroxide and sodium hydroxide solutions a...
Text Solution
|
- What happens when ammonium hydroxide and sodium hydroxide solutions a...
Text Solution
|
- What happens when ammonium hydroxide and sodium hydroxide solutions a...
Text Solution
|
- What happens when ammonium hydroxide and sodium hydroxide solutions a...
Text Solution
|
- What happens when ammonium hydroxide and sodium hydroxide solutions a...
Text Solution
|
- What happens when ammonium hydroxide and sodium hydroxide solutions a...
Text Solution
|