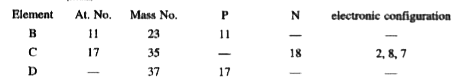Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SPECIMEN PAPER 2-SECTION -II
- (i) Study the above table and fill in the blanks. (ii) What is the r...
Text Solution
|
- Give reasons : Sodium is kept in an inert solvent.
Text Solution
|
- Give reasons : Hydrogen is collected by downward displacement of wat...
Text Solution
|
- Give reasons : Hydrogen is not prepared by reacting conc H(2)SO(4) w...
Text Solution
|
- Give reasons : Conc. sulphuric acid is used as drying agent.
Text Solution
|
- Give reasons : Detergents are better than soap.
Text Solution
|
- Give the test of the gas evolved when dilute sulphuric acid is added t...
Text Solution
|
- Give the test of the gas evolved when dilute sulphuric acid is added t...
Text Solution
|
- Give the test of the gas evolved when dilute sulphuric acid is added t...
Text Solution
|
- Give the test of the gas evolved when dilute sulphuric acid is added t...
Text Solution
|
- Give an example of a reaction where heat is released.
Text Solution
|
- Name the substances responsible for permanent hardness
Text Solution
|
- Explain one method of removing permanent hardness.
Text Solution
|
- Complete the table by writing the following as basic and acidic radica...
Text Solution
|
- State the cause of acid rain and mention its impact.
Text Solution
|
- Explain why : Fused CaCl2 or conc. H2SO4 is used in a desiccator.
Text Solution
|
- Explain why? conc. sulphuric acid should be stoppered.
Text Solution
|
- Write balanced equations for the following reactions. Water is react...
Text Solution
|
- Write balanced equations for the following reactions. Steam is passe...
Text Solution
|
- Write balanced equations for the following reactions. Ammonium dichr...
Text Solution
|
- Write balanced equations for the following reactions. Lead reacts wi...
Text Solution
|