Topper's Solved these Questions
STUDY OF COMPOUNDS - NITRIC ACID
ICSE|Exercise ADDITIONAL QUESTIONS |21 VideosSTUDY OF COMPOUNDS - NITRIC ACID
ICSE|Exercise UNIT TEST PAPER 7 C - NITRIC ACID |27 VideosSTUDY OF COMPOUNDS - HYDROGEN CHLORIDE
ICSE|Exercise UNIT TEST PAPER 7A - HYDROGEN CHLORIDE|28 VideosSTUDY OF COMPOUNDS - STUDY OF HYDROGEN CHLORIDE (HCL)
ICSE|Exercise QUESTIONS FOR PRACTICE|36 Videos
Similar Questions
Explore conceptually related problems
ICSE-STUDY OF COMPOUNDS - NITRIC ACID -UNIT TEST PAPER 7 C - NITRIC ACID
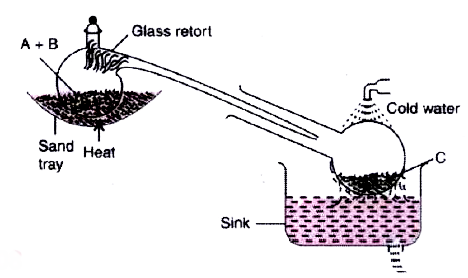
- The figure given below illustrates the apparatus used in the laborator...
Text Solution
|
- Select the letters A,B,C,D or E , which form the gaseous products of t...
Text Solution
|
- Select the letters A,B,C,D or E , which form the gaseous products of t...
Text Solution
|
- Select the letters A,B,C,D or E , which form the gaseous products of t...
Text Solution
|
- Select the letters A,B,C,D or E , which form the gaseous products of t...
Text Solution
|
- Select the letters A,B,C,D or E , which form the gaseous products of t...
Text Solution
|
- Select the correct word from the list in bracket to complete each stat...
Text Solution
|
- Select the correct word from the list in bracket to complete each stat...
Text Solution
|
- Select the correct word from the list in bracket to complete each stat...
Text Solution
|
- Select the correct word from the list in bracket to complete each stat...
Text Solution
|
- Select the correct word from the list in bracket to complete each stat...
Text Solution
|
- Give balanced equations for the following conversion A to E. Co...
Text Solution
|
- Give balanced equations for the following conversion D to E. Su...
Text Solution
|
- Name the oxidised product when Sulphur is reacted with Nitric acid
Text Solution
|
- Name the oxidised product when the following 1 to 5 react with nitric ...
Text Solution
|
- Name the oxidised product when the following 1 to 5 react with nitric ...
Text Solution
|
- Name the oxidised product when the following 1 to 5 react with nitric ...
Text Solution
|
- Name the oxidised product when Carbon reacts with Nitric acid
Text Solution
|
- Give reasons for the following : Nitric acid is not manufactured fr...
Text Solution
|
- Give reasons for the following : Nitric acid affects the skin if it ...
Text Solution
|
- Give reasons for the following : The yellow colour of nitric acid ...
Text Solution
|