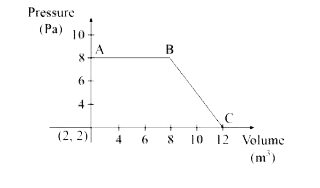
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The magnitude of work done by a gas that undergoes a reversible expans...
Text Solution
|
- A gas undergoes a cyclic process ABCDA as shown in the figure. The par...
Text Solution
|
- The work done in an isothermal reversible expansion of n moles of a ga...
Text Solution
|
- Which expression is correct for the work done in adiabatic reversible ...
Text Solution
|
- A gas undergoes expansion according to the following graph. Calculate ...
Text Solution
|
- In an isothennal reversible expansion ofim ideal gas, work done is
Text Solution
|
- Work done in a reversible expansion is
Text Solution
|
- In a reversible isothermal expansion of ideal gas, the work done is gi...
Text Solution
|
- What is the work done in the free expansion of an ideal gas in reversi...
Text Solution
|