Text Solution
Verified by Experts
Topper's Solved these Questions
SOLID STATE
ICSE|Exercise WORKED OUT EXAMPLE(Type III. Calculation of Avogadro.s Number)|1 VideosSOLID STATE
ICSE|Exercise WORKED OUT EXAMPLE(Type IV. Calculation of Atomic Mass and Number of Atoms in a Given Mass.)|2 VideosSOLID STATE
ICSE|Exercise WORKED OUT EXAMPLE(Type I. Calculation of Density of Unit Cell)|1 VideosSELF ASSESSMENT PAPER-8
ICSE|Exercise QUESTIONS|11 VideosSOLUTIONS
ICSE|Exercise MULTIPLE CHOICE QUESTIONS (Assertion and Reason based questions)|10 Videos
Similar Questions
Explore conceptually related problems
ICSE-SOLID STATE-WORKED OUT EXAMPLE(Type II. Calculation of Edge length, Interionic distances and volume of unit cell from Density.)
- Niobium crystallizes in body centred cubic structure. If its density i...
Text Solution
|
- Silver metal crystallizes with a face centred cubic lattice. The lengt...
Text Solution
|
- Lithium metal has a body centred cubic structure. Its density is 0.53g...
Text Solution
|
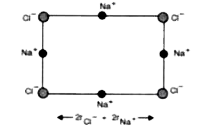
- What is the distance between Na^(+) and Cl^(-) ions in NaCl crystal if...
Text Solution
|