Text Solution
Verified by Experts
Topper's Solved these Questions
P-BLOCK ELEMENTS
ICSE|Exercise EXERCISE (PART-II)(DESCRIPTIVE QUESTIONS)(SHORT ANSWER QUESTIONS)|67 VideosP-BLOCK ELEMENTS
ICSE|Exercise EXERCISE (PART-II)(DESCRIPTIVE QUESTIONS)(LONG ANSWER QUESTIONS)|65 VideosP-BLOCK ELEMENTS
ICSE|Exercise EXERCISE (PART-I)(MATCH THE FOLLOWING)|1 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
ICSE|Exercise ISC EXAMINATION QUESTIONS ( PART-II DESCRIPTIVE QUESTIONS)|33 VideosPOLYMERS
ICSE|Exercise ISC EXAMINATION QUESTIONS |10 Videos
Similar Questions
Explore conceptually related problems
ICSE-P-BLOCK ELEMENTS-EXERCISE (PART-II)(DESCRIPTIVE QUESTIONS)(VERY SHORT ANSWER QUESTIONS)
- Name the series of salts that are formed by orthophosphoric acid with ...
Text Solution
|
- What is the state of hybridization of nitrogen in NO3^(-) ion ?
Text Solution
|
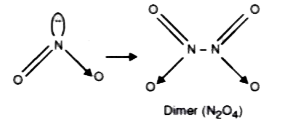
- NO2 readily forms a dimer. Explain.
Text Solution
|
- What is laughing gas ? How is it prepared?
Text Solution
|
- On being slowly passed through water, PH3 forms bubbles but NH3 dissol...
Text Solution
|
- Why does H3PO3 act as a reducing agent but H3PO4 does not?
Text Solution
|
- Mention one use of hydrazine.
Text Solution
|
- Basicity of H3PO2, H3PO3 and H3PO4 is not the same. Explain
Text Solution
|
- What are chalcogens ? Why are group 16 elements called chalcogens ?
Text Solution
|
- What is the outer shell electronic configuration of chalcogens?
Text Solution
|
- Name the allotropes of oxygen.
Text Solution
|
- Which element of group 16 has maximum tendency for catenation ?
Text Solution
|
- Ionisation enthalpy of nitrogen is more than oxygen because of
Text Solution
|
- What is hypo?
Text Solution
|
- Sodium thiosulfate is a salt of which acid ?
Text Solution
|
- Give one method of preparation of sodium thiosulfate.
Text Solution
|
- Which form of sulphur shows paramagnetic behaviour ?
Text Solution
|
- What are allotropes of sulfur?
Text Solution
|
- What do you understand by (a) inert pair effect (b) allotropy and (c...
Text Solution
|
- What is oleum ? What is the oxidation state of sulfur in it?
Text Solution
|