Text Solution
Verified by Experts
Topper's Solved these Questions
P-BLOCK ELEMENTS
ICSE|Exercise EXERCISE (PART-II)(DESCRIPTIVE QUESTIONS)(SHORT ANSWER QUESTIONS)|67 VideosP-BLOCK ELEMENTS
ICSE|Exercise EXERCISE (PART-II)(DESCRIPTIVE QUESTIONS)(LONG ANSWER QUESTIONS)|65 VideosP-BLOCK ELEMENTS
ICSE|Exercise EXERCISE (PART-I)(MATCH THE FOLLOWING)|1 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
ICSE|Exercise ISC EXAMINATION QUESTIONS ( PART-II DESCRIPTIVE QUESTIONS)|33 VideosPOLYMERS
ICSE|Exercise ISC EXAMINATION QUESTIONS |10 Videos
Similar Questions
Explore conceptually related problems
ICSE-P-BLOCK ELEMENTS-EXERCISE (PART-II)(DESCRIPTIVE QUESTIONS)(VERY SHORT ANSWER QUESTIONS)
- Draw the structure of xenon oxyfluoride which is isoelectronic with IF...
Text Solution
|
- Fluorine provides the largest variety of interhalogen compounds among ...
Text Solution
|
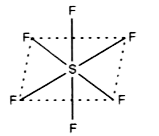
- Draw and name the molecular shape of SF6.
Text Solution
|
- Out of H(2)O which one has higher bond angle and why?
Text Solution
|
- Name one ion whose central atom has sp^3 d^3 type of hybrid orbitals.
Text Solution
|
- What type of hybridization is associated with N in NH3 ? What is th...
Text Solution
|
- The bond energy of fluorine is more than that of chlorine.
Text Solution
|
- Account for the following: Tendency to show -2 oxidation state dimi...
Text Solution
|
- Which is the strongest oxidising agent among CIO(4)^(-),BrO(4)^(-) an...
Text Solution
|
- Why is H2S more acidic than H2O ?
Text Solution
|
- Which type of hybridisation explains the trigonal bipyramidal shape of...
Text Solution
|
- Draw the structure of BrO(4)^(-)
Text Solution
|
- Whys is F(2) a stronger oxidising agent than Cl(2) ?
Text Solution
|
- Write the chemical equation for the reactions which occur when sodium ...
Text Solution
|
- Draw the structure of XeF4 molecule.
Text Solution
|
- Complete the following chemical equation : XeF4 + H2O rarr
Text Solution
|
- Write one chemical equation to show that chlorine gas can be obtained ...
Text Solution
|
- Write one chemical equation to show that SO2 acts as a reducing agent...
Text Solution
|
- Write one chemical equation that conc. H2SO4 is a strong oxidising age...
Text Solution
|
- Give a chemical equation or name of the reaction to show that sodium c...
Text Solution
|