Text Solution
Verified by Experts
Topper's Solved these Questions
P-BLOCK ELEMENTS
ICSE|Exercise EXERCISE (PART-II)(DESCRIPTIVE QUESTIONS)(SHORT ANSWER QUESTIONS)|67 VideosP-BLOCK ELEMENTS
ICSE|Exercise EXERCISE (PART-II)(DESCRIPTIVE QUESTIONS)(LONG ANSWER QUESTIONS)|65 VideosP-BLOCK ELEMENTS
ICSE|Exercise EXERCISE (PART-I)(MATCH THE FOLLOWING)|1 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
ICSE|Exercise ISC EXAMINATION QUESTIONS ( PART-II DESCRIPTIVE QUESTIONS)|33 VideosPOLYMERS
ICSE|Exercise ISC EXAMINATION QUESTIONS |10 Videos
Similar Questions
Explore conceptually related problems
ICSE-P-BLOCK ELEMENTS-EXERCISE (PART-II)(DESCRIPTIVE QUESTIONS)(VERY SHORT ANSWER QUESTIONS)
- Whys is F(2) a stronger oxidising agent than Cl(2) ?
Text Solution
|
- Write the chemical equation for the reactions which occur when sodium ...
Text Solution
|
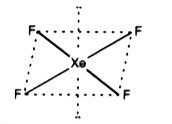
- Draw the structure of XeF4 molecule.
Text Solution
|
- Complete the following chemical equation : XeF4 + H2O rarr
Text Solution
|
- Write one chemical equation to show that chlorine gas can be obtained ...
Text Solution
|
- Write one chemical equation to show that SO2 acts as a reducing agent...
Text Solution
|
- Write one chemical equation that conc. H2SO4 is a strong oxidising age...
Text Solution
|
- Give a chemical equation or name of the reaction to show that sodium c...
Text Solution
|
- Find the oxidation number of sulfur in peroxomono-sulfuric acid.
Text Solution
|
- Write the formula of the noble gas species that is isostructural with ...
Text Solution
|
- Draw the structure of peroxodisulfuric acid.
Text Solution
|
- Which is more stable PCl5 or PCl3 ?
Text Solution
|
- Write the structural formula of PCl5(s).
Text Solution
|
- What type of hybridization is involved in PCI5 ?
Text Solution
|
- Why is sulfurous acid a reducing agent ?
Text Solution
|
- Why do hydrides of oxygen and sulfur differ in physical state?
Text Solution
|
- Which halogen has highest value of electron gain enthalpy?
Text Solution
|
- Write the chemical equation for the following: Ca(3)(PO4)2 + SiO(2) ...
Text Solution
|
- Why is nitrous acid oxidant as well as reductant ?
Text Solution
|
- Which is least basic ? SbH(3)PH3,NH3,AsH3
Text Solution
|