Text Solution
Verified by Experts
Topper's Solved these Questions
P-BLOCK ELEMENTS
ICSE|Exercise EXERCISE (PART-II)(DESCRIPTIVE QUESTIONS)(SHORT ANSWER QUESTIONS)|67 VideosP-BLOCK ELEMENTS
ICSE|Exercise EXERCISE (PART-II)(DESCRIPTIVE QUESTIONS)(LONG ANSWER QUESTIONS)|65 VideosP-BLOCK ELEMENTS
ICSE|Exercise EXERCISE (PART-I)(MATCH THE FOLLOWING)|1 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
ICSE|Exercise ISC EXAMINATION QUESTIONS ( PART-II DESCRIPTIVE QUESTIONS)|33 VideosPOLYMERS
ICSE|Exercise ISC EXAMINATION QUESTIONS |10 Videos
Similar Questions
Explore conceptually related problems
ICSE-P-BLOCK ELEMENTS-EXERCISE (PART-II)(DESCRIPTIVE QUESTIONS)(VERY SHORT ANSWER QUESTIONS)
- Why is nitrous acid oxidant as well as reductant ?
Text Solution
|
- Which is least basic ? SbH(3)PH3,NH3,AsH3
Text Solution
|
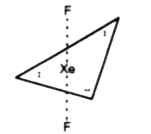
- Draw the structure of XeF(2)
Text Solution
|
- Among the noble gases only xenon is well known to form chemical compou...
Text Solution
|
- Assign reason for the following: In solid state, PCI5 , behaves as an ...
Text Solution
|
- Are all the five bonds in PCl(5) molecule equivalent? Justify your ans...
Text Solution
|
- Complete the following reactions : (i) P4 + SOCl2 rarr
Text Solution
|
- Complete the following reactions : (ii) CH3COOH + PCl5 rarr
Text Solution
|
- What happens when (write balanced chemical equations): Ammonia is ...
Text Solution
|
- In which one of the two structures, NO(2)^(+) and NO(2)^(-) the bond a...
Text Solution
|
- Why is the bond angle in PH(3) molecule lesser than that in NH(3) mole...
Text Solution
|
- Answer the following : (i) Which neutral molecule would be isoelectr...
Text Solution
|
- Why is Bi(V) a stronger oxidant than Sb(V) ?
Text Solution
|
- Why is red phosporus less reactive than white phosphorus?
Text Solution
|
- In the ring test for identification of nitrate ion, what is the formul...
Text Solution
|
- Write the balanced equation for complete hydrolysis of XeF6.
Text Solution
|
- In what way can it be proved that PH(3) is basic in nature?
Text Solution
|
- Draw the structure of O(3) molecule.
Text Solution
|
- Explain the following observations : (i) Fluorine does not exhibit a...
Text Solution
|
- Nitrogen is relatively inert as compared to phosphorus. Why ?
Text Solution
|