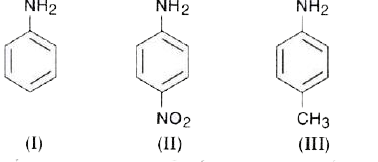
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING NITROGEN
ICSE|Exercise EXERCISE (PART-II DESCRIPTIVE QUESTIONS- VERY SHORT ANSWER QUESTIONS )|89 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
ICSE|Exercise EXERCISE (PART-II DESCRIPTIVE QUESTIONS- SHORT ANSWER QUESTIONS )|92 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
ICSE|Exercise ISC EXAMINATION QUESTIONS ( PART-II DESCRIPTIVE QUESTIONS)|33 VideosISC QUESTION PAPER
ICSE|Exercise PART-II(SECTION-B)|74 VideosP-BLOCK ELEMENTS
ICSE|Exercise SOURCE BASED QUESTIONS|10 Videos
Similar Questions
Explore conceptually related problems
ICSE-ORGANIC COMPOUNDS CONTAINING NITROGEN-EXERCISE (PART-I OBJECTIVE QUESTIONS)
- The best reagent for converting, 2-phenylpropanamide into 1-phenyletha...
Text Solution
|
- Hofmann bromide degradation reaction is shown by
Text Solution
|
- The correct increasing order of basic strength for the following compo...
Text Solution
|
- Methylamine reacts with HNO2 to form
Text Solution
|
- The gas evolved when methylamine reacts with nitrous acid is….
Text Solution
|
- In the nitration of benzene using a mixture of conc. H2SO4 and conc. H...
Text Solution
|
- Reduction of aromatic nitro compounds using Fe and HCl gives .
Text Solution
|
- The most reactive amine towards dilute hydrochloric acid is .
Text Solution
|
- Acid anhydrides on reaction with primary amine gives…
Text Solution
|
- The reaction Aroverset(+)N(2)Cl^(-) overset(Cu//HCl)to ArCl +N(2) + ...
Text Solution
|
- Best method for preparing primary amines from alkyl halides without ch...
Text Solution
|
- Which of the following compounds will not undergo azo coupling reactio...
Text Solution
|
- Which of the following compounds is the weakest Bronsted base?
Text Solution
|
- Among the following amines, the strongest Bronsted base is….
Text Solution
|
- The correct decreasing order of basic strength of the following specie...
Text Solution
|
- Which of the following should be most volatile ?
Text Solution
|
- Which of the following methods of preparation of amines will not give ...
Text Solution
|
- Gabrial synthesis is used for the preparation of
Text Solution
|
- Nitrosoamines are formed byunderline(" primary amines").
Text Solution
|
- Pure primary amines underline("can be prepared ")by the action of ammo...
Text Solution
|