Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING NITROGEN
ICSE|Exercise EXERCISE (PART-II DESCRIPTIVE QUESTIONS- LONG ANSWER QUESTIONS )|57 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
ICSE|Exercise ISC EXAMINATION QUESTIONS ( PART-I OBJECTIVE QUESTIONS)|7 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
ICSE|Exercise EXERCISE (PART-II DESCRIPTIVE QUESTIONS- VERY SHORT ANSWER QUESTIONS )|89 VideosISC QUESTION PAPER
ICSE|Exercise PART-II(SECTION-B)|74 VideosP-BLOCK ELEMENTS
ICSE|Exercise SOURCE BASED QUESTIONS|10 Videos
Similar Questions
Explore conceptually related problems
ICSE-ORGANIC COMPOUNDS CONTAINING NITROGEN-EXERCISE (PART-II DESCRIPTIVE QUESTIONS- SHORT ANSWER QUESTIONS )
- Write reactions of the final alkylation product of aniline with excess...
Text Solution
|
- Write structures of different isomers corresponding to the molecular f...
Text Solution
|
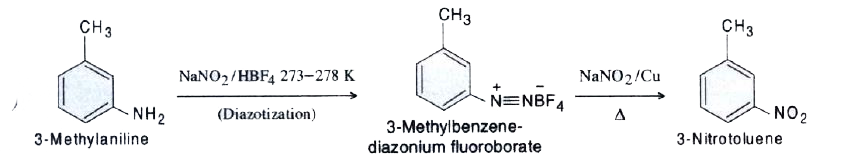
- Convert: 3-Methylaniline into 3-nitrotoluene
Text Solution
|
- Convert: Aniline into 1, 3,5-tribromobenzene.
Text Solution
|
- How will you bring about the following conversions ? Ethyl bromide ...
Text Solution
|
- How will you bring about the following conversions ? Methylamine to ...
Text Solution
|
- Explain Libermann's nitrosoamine reaction.
Text Solution
|
- What is meant by : Acylation
Text Solution
|
- What is meant by : benzoylation ?
Text Solution
|
- Like ammonia, amines combine with metal ions to form coordination comp...
Text Solution
|
- How do primary secondary and tertiary amines react with nitrous acid ?
Text Solution
|
- Do as directed : (i) Arrange the following compounds in the increasi...
Text Solution
|
- Explain how does the presence or absence of hydrogen on nitrogen of am...
Text Solution
|
- Describe the following processes with an example in each case : Pro...
Text Solution
|
- Describe the following processes with an example in each case : Ace...
Text Solution
|
- Write one chemical equation each to examplify the following reactions ...
Text Solution
|
- Write the chemical equations involeved in the following reactions: ...
Text Solution
|
- Explain the following: Aniline is less basic than ammonia.
Text Solution
|
- Explain the following: Aniline is a weaker base than ethylamine.
Text Solution
|
- Arrange the following sets in order of their basic strength: Ethyla...
Text Solution
|