Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-EQUILIBRIUM -NCERT TEXT-BOOK EXERCISE
- What will be the conjugate bases for the Brönsted acids: HF,H(2)SO(4) ...
Text Solution
|
- Write the conjugate acids for the following Bronsted bases : NH(2)^(-)...
Text Solution
|
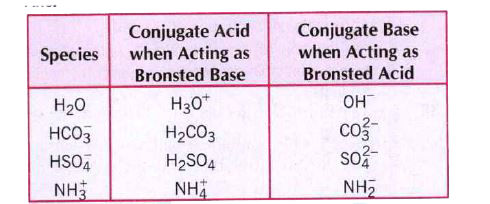
- The species: H(2)O, HCO(3)^(-), HSO(4)^(-) and NH(3) can act both as B...
Text Solution
|
- Classify the following species into Lewis acids and Lewis bases and sh...
Text Solution
|
- Classify the following species into Lewis acid and Lewis base. And sh...
Text Solution
|
- Classify the following species into Lewis acids and Lewis bases and sh...
Text Solution
|
- Classify the following species into Lewis acids and Lewis bases and sh...
Text Solution
|
- The concentration of hydrogen ion in a sample of soft drink is 3.8 xx ...
Text Solution
|
- The pH of a sample of vinegar is 3.76. Calculate the concentration of ...
Text Solution
|
- The ionization constant of HF, HCOOH and HCN at 298K are 6.8 xx 10^(-4...
Text Solution
|
- The ionization constant of phenol is 1.0 xx 10^(-10). What is the conc...
Text Solution
|
- The first ionization constant of H(2)S is 9.1 xx 10^(-8). Calculate th...
Text Solution
|
- The ionization constant of acetic acid is 1.74 xx 10^(-5). Calculate t...
Text Solution
|
- It has been found that the pH of a 0.01M solution of an organic acid i...
Text Solution
|
- Assuming complete dissociation , calculate the pH of the following s...
Text Solution
|
- Assuming complete dissociation, calculate the pH of the following solu...
Text Solution
|
- Assuming complete dissociation, calculate the pH of the following solu...
Text Solution
|
- Assuming complete dissociation, calculate the pH of the following solu...
Text Solution
|
- Calculate the pH of the following solutions : 2 g of TIOH dissolved...
Text Solution
|
- Calculate the pH of the following solutions : 0.3 g of Ca(OH)2 dis...
Text Solution
|