Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
ICSE|Exercise REVIEW EXERCISES|176 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
ICSE|Exercise VERY SHORT ANSWER TYPE QUESTIONS|87 VideosHYDROGEN
ICSE|Exercise NCERT TEXT-BOOK EXERCISE (With Hints and Solutions)|53 VideosREDOX REACTIONS (OXIDATION AND REDUCTION)
ICSE|Exercise NCERT TEXT-BOOK. EXERCISES ((With Hints and Solutions)|69 Videos
Similar Questions
Explore conceptually related problems
ICSE-ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES -NCERT TEXT-BOOK EXERCISES (WITH HINTS AND SOLUTIONS )
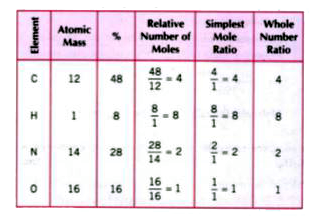
- A sample of 0.50 g of an organic compound was treated according to Kje...
Text Solution
|
- What are hybridisation states of each carbon atom in the following com...
Text Solution
|
- Indicate the sigma and pi bonds in the following molecules: C6 H6 ,...
Text Solution
|
- Write bond line formulas for : 2, 3-dimethyl butanal, Heptan-4-one.
Text Solution
|
- Give the I.U.P.A.C. names of the following compounds:
Text Solution
|
- Give the I.U.P.A.C. names of the following compounds:
Text Solution
|
- Give the I.U.P.A.C. names of the following compounds:
Text Solution
|
- Give the I.U.P.A.C. names of the following compounds:
Text Solution
|
- Give the I.U.P.A.C. names of the following compounds:
Text Solution
|
- Give the I.U.P.A.C. names of the following compounds: Cl2 CHCH2 OH
Text Solution
|
- Which of the following represents the correct I.U.P.A.C. name for the ...
Text Solution
|
- Which of the following represents the correct I.U.P.A.C. name for the ...
Text Solution
|
- Which of the following represents the correct I.U.P.A.C. name for the ...
Text Solution
|
- Which of the following represents the correct I.U.P.A.C. name for the ...
Text Solution
|
- Draw formulas for the first five members of each homologous series beg...
Text Solution
|
- Draw formulas for the first five members of each homologous series beg...
Text Solution
|
- Draw formulas for the first five members of each homologous series beg...
Text Solution
|
- Give condensed and bond line structural formulas and identify the func...
Text Solution
|
- Give condensed and bond line structural formulas and identify the func...
Text Solution
|
- Give condensed and bond line structural formulas and identify the func...
Text Solution
|
- Identify the functional groups is the following compounds:
Text Solution
|