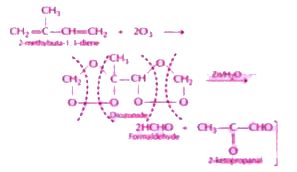
Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
ICSE|Exercise VERY SHORT ANSWER TYPE QUESTIONS|87 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
ICSE|Exercise SHORT ANSWER TYPE QUESTIONS|110 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
ICSE|Exercise NCERT TEXT-BOOK EXERCISES (WITH HINTS AND SOLUTIONS )|72 VideosHYDROGEN
ICSE|Exercise NCERT TEXT-BOOK EXERCISE (With Hints and Solutions)|53 VideosREDOX REACTIONS (OXIDATION AND REDUCTION)
ICSE|Exercise NCERT TEXT-BOOK. EXERCISES ((With Hints and Solutions)|69 Videos
Similar Questions
Explore conceptually related problems
ICSE-ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES -REVIEW EXERCISES
- Establish the structure of a hydrocarbon C5 H(10) from the following ...
Text Solution
|
- A hydrocarbon (V.D. = 27) containing C = 88.88% decolourised KMnO4 sol...
Text Solution
|
- An organic compound (A) having V.D. = 30 contains C = 60.0% and H= 13%...
Text Solution
|
- An organic compound E (C5 H8) on hydrogenation gives compound F (C5 H(...
Text Solution
|
- An aliphatic hydrocarbon (A) of molecular weight 58 yields on chlorina...
Text Solution
|
- Arrange the following in the order as mentioned : (CH3)3 C-(CH3)2 CH...
Text Solution
|
- Arrange the following in the order as mentioned : -CN, -CI, -OH, -NO2...
Text Solution
|
- Arrange the following in the order as mentioned : overset(+ )CH3 CH3 ...
Text Solution
|
- Arrange the following in the order as mentioned : 1^@ , 2^@ , 3^@ f...
Text Solution
|
- What are the main points of difference between inductive and electrome...
Text Solution
|
- Explain the l-effect leads to the development of partial charges ...
Text Solution
|
- Explain the Hyperconjugation effect is also termed as 'no bond reso...
Text Solution
|
- Explain the overset(* )CH3 is more reactive than CH3 CH2 free radic...
Text Solution
|
- Explain the The carbanions are very reactive species although their...
Text Solution
|
- Explain the BF3 acts as an electrophile.
Text Solution
|
- Explain the CH3 NH2 acts as a nucleophile.
Text Solution
|
- Why is in the presence of diethyl peroxide, the addition of HBr to pro...
Text Solution
|
- Why is chloroacetic acid is a stronger acid as compared to acetic acid...
Text Solution
|
- Explain the (CH3)3 C^(-) is less stable than bar(C) H3.
Text Solution
|
- Explain the A singlet carbene has a bent structure.
Text Solution
|