A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
ICSE|Exercise TRUE OR FALSE TYPE QUESTIONS|50 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
ICSE|Exercise FILL IN THE BLANK TYPE QUESTIONS |46 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
ICSE|Exercise ESSAY ( LONG ANSWER ) TYPE QUESTIONS|42 VideosHYDROGEN
ICSE|Exercise NCERT TEXT-BOOK EXERCISE (With Hints and Solutions)|53 VideosREDOX REACTIONS (OXIDATION AND REDUCTION)
ICSE|Exercise NCERT TEXT-BOOK. EXERCISES ((With Hints and Solutions)|69 Videos
Similar Questions
Explore conceptually related problems
ICSE-ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES -OBJECTIVE ( MULTIPLE CHOICE ) TYPE QUESTIONS
- Which of the following statements is not correct?
Text Solution
|
- Consider the following conformations. Which of the following statement...
Text Solution
|
- The structure shows
Text Solution
|
- Which of the following has an asymmetric carbon atom ? (a)CH3 - CH2 -...
Text Solution
|
- The compound which does not exhibit optical isomerism is
Text Solution
|
- The correct statements about the compounds A, B and C is
Text Solution
|
- How many optically active stereoisomers are possible for butane-2, 3-d...
Text Solution
|
- During debromination of meso-dibromobutane, the major compound formed ...
Text Solution
|
- Which one of the following compounds will show geometrical isomerism ?
Text Solution
|
- Out of the following, the alkene that exhibits optical isomerism is ...
Text Solution
|
- Identify the compound that exhibits tautomerism: 2-butene lactic...
Text Solution
|
- How many chiral compounds are possible on monochlorination of 2-methyl...
Text Solution
|
- Which branched chain isomer of the hydrocarbon with molecular mass 72u...
Text Solution
|
- Which of the following acids does not exhibit optical isomerism?
Text Solution
|
- Lassaigne's test is used in the qualitative analysis to detect
Text Solution
|
- A compound which does not give positive test for nitrogen is: urea ...
Text Solution
|
- For detection of sulphur in an organic compound, sodium nitroprusside ...
Text Solution
|
- In sodium fusion test of organic compounds, the nitrogen of an organic...
Text Solution
|
- The Beilstein test for organic compounds is used to detect
Text Solution
|
- In the Duma's method for estimating nitrogen in an organic compound, t...
Text Solution
|
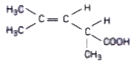 shows
shows