Topper's Solved these Questions
STUDY OF COMPOUNDS - AMMONIA
ICSE|Exercise ADDITIONAL QUESTIONS|30 VideosSTUDY OF COMPOUNDS - AMMONIA
ICSE|Exercise UNIT TEST PAPER 7B - AMMONIA |22 VideosSTUDY OF COMPOUNDS - AMMONIA
ICSE|Exercise UNIT TEST PAPER 7B - AMMONIA |22 VideosSTUDY OF ACIDS, BASES AND SALTS
ICSE|Exercise ASSERTION AND REASON BASED QUESTIONS|5 VideosSTUDY OF COMPOUNDS - HYDROGEN CHLORIDE
ICSE|Exercise UNIT TEST PAPER 7A - HYDROGEN CHLORIDE|28 Videos
Similar Questions
Explore conceptually related problems
ICSE-STUDY OF COMPOUNDS - AMMONIA -QUESTIONS
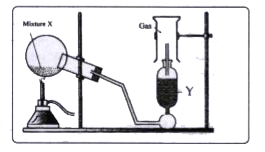
- The diagram shows an experimental set up for the laboratory preparatio...
Text Solution
|
- The diagram shows an experimental set up for the laboratory preparatio...
Text Solution
|
- The diagram shows an experimental set up for the laboratory preparatio...
Text Solution
|
- The diagram shows an experimental set up for the laboratory preparatio...
Text Solution
|
- Write balanced chemical equation for the following: Chlorine reacts wi...
Text Solution
|
- What would you observe in the following case ? Water is added to the...
Text Solution
|
- Name the gas in the following: The gas produced when excess ammonia re...
Text Solution
|
- Some word/words are missing in the following statement. You are requir...
Text Solution
|
- Give balanced equation for the reaction : Ammonua & oxygen in the pres...
Text Solution
|
- The following questions are based on the preparation of ammonia gas in...
Text Solution
|
- State one appropriate observation for the following: Excess of chlorin...
Text Solution
|
- Nitrogen gas can be obtained by heating : A: Ammonium nitrate B: Ammon...
Text Solution
|
- State two observations for : NH(4)OH soln . Is added to zinc nitrate s...
Text Solution
|
- Give a balanced equation for : Reduction of hot Copper (II) oxide to c...
Text Solution
|
- State the -i] Temperature ii]Catalyst used in the Haber 's process for...
Text Solution
|
- Identify : An alkaline gas which produces dense white fumes when react...
Text Solution
|
- Fill in the blank from the choices given within brackets : Ammonia g...
Text Solution
|
- Write balanced equation for : Action of warm water om magnesium nitrid...
Text Solution
|
- Distinguish between the following pairs of compounds using the test gi...
Text Solution
|
- State your observation :alcium hydroxide is heated with ammonium chlor...
Text Solution
|