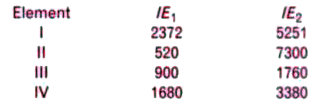Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
ICSE|Exercise REVIEW EXERCISES|29 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
ICSE|Exercise VERY SHORT ANSWER TYPE QUESTIONS|25 VideosCHEMICAL THERMODYNAMICS
ICSE|Exercise NCERT TEXT-BOOK. EXERCISES|22 VideosENVIRONMENTAL CHEMISTRY
ICSE|Exercise NCERT TEXT-BOOK EXERCISE|20 Videos
Similar Questions
Explore conceptually related problems
ICSE-CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES-NCERT TEXT-BOOK. EXERCISE (With Hints and Solutions)
- The first (IE(1)) and the second (IE(2)) ionisation energies (kJ mol^(...
Text Solution
|
- What is the basic theme of organisation in the periodic table?
Text Solution
|
- Which important property did Mendeleev use to classify the elements in...
Text Solution
|
- What is the basic difference in approach between the Mendeleev’s Perio...
Text Solution
|
- On the basis of quantum numbers, justify that the sixth period of the ...
Text Solution
|
- In terms of period and group where would you locate the element with Z...
Text Solution
|
- Atomic number of element present in the third period and seventeenth g...
Text Solution
|
- Which element do you think would have been named by Lawrence Berkley...
Text Solution
|
- Which element do you think would have been named by Seaborg's group...
Text Solution
|
- Why do elements in the same group have similar physical and chemical p...
Text Solution
|
- What does atomic radius and ionic radius really mean to you?
Text Solution
|
- How do atomic radii vary in a period and in a group? How do you explai...
Text Solution
|
- What do you understand by isoelectronic species? Name a species that w...
Text Solution
|
- What do you understand by isoelectronic species? Name a species that w...
Text Solution
|
- What do you understand by isoelectronic species? Name a species that w...
Text Solution
|
- What do you understand by isoelectronic species? Name a species that w...
Text Solution
|
- Consider the following species: N^(3-),O^(2-),F^(-),Na^(+),Mg^(2+) a...
Text Solution
|
- What is common between given cations and anions,O^(2-)F^(-),Na^(+),Mg^...
Text Solution
|
- Explain why cation are smaller and anions larger in radii than their p...
Text Solution
|
- What is the significance of the terms — ‘isolated gaseous atom’ and ‘g...
Text Solution
|
- Energy of an electron in the ground state of the hydrogen atom is -2.1...
Text Solution
|