Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES-NCERT TEXT-BOOK. EXERCISE (With Hints and Solutions)
- Assign the position of the element having outer electronic configurati...
Text Solution
|
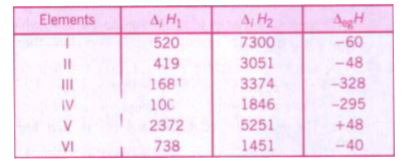
- The first (Delta(i), H(1)) and the second (Delta, H(2)) ionisation ent...
Text Solution
|
- The first (Delta(i), H(1)) and the second (Delta, H(2)) ionisation ent...
Text Solution
|
- The first (Delta(i), H(1)) and the second (Delta, H(2)) ionisation ent...
Text Solution
|
- The first (Delta(i), H(1)) and the second (Delta, H(2)) ionisation ent...
Text Solution
|
- Calculate the work done when 1.0 mole water at 373K vaporizes against ...
Text Solution
|
- The first (Delta(i), H(1)) and the second (Delta, H(2)) ionisation ent...
Text Solution
|
- Predict the formulae of the stable binary compounds that would be form...
Text Solution
|
- Predict the formulae of the stable binary compounds that would be form...
Text Solution
|
- Predict the formulae of the stable binary compounds that would be form...
Text Solution
|
- Predict the formulae of the stable binary compounds that would be form...
Text Solution
|
- Predict the formulae of the stable binary compounds that would be form...
Text Solution
|
- Predict the formulae of the stable binary compounds that would be form...
Text Solution
|
- In the modern periodic table, the period indicates the value of :
Text Solution
|
- Which of the following statements related to the modern periodic table...
Text Solution
|
- Anything that influences the valence electrons will affect the chemist...
Text Solution
|
- The size of isoelectronic species - F^(-), Ne and Na^(+) is affected b...
Text Solution
|
- Which one of the following statements is incorrect in relation to ioni...
Text Solution
|
- Considering the elements B, Al, Mg, and K, the correct order of their ...
Text Solution
|
- Considering the elements B, C, N, F, and Si, the correct order of thei...
Text Solution
|