Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL THERMODYNAMICS
ICSE|Exercise REVIEW EXERCISES (Fill in the blanks )|6 VideosCHEMICAL THERMODYNAMICS
ICSE|Exercise VERY SHORT ANSWER TYPE QUESTIONS |56 VideosCHEMICAL THERMODYNAMICS
ICSE|Exercise NCERT TEXT-BOOK. EXERCISES|22 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
ICSE|Exercise NCERT TEXT-BOOK EXERCISES ( WITH HINTS AND SOLUTIONS)|47 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
ICSE|Exercise NCERT TEXT-BOOK. EXERCISE (With Hints and Solutions)|63 Videos
Similar Questions
Explore conceptually related problems
ICSE-CHEMICAL THERMODYNAMICS -REVIEW EXERCISES
- For a hypothetical reaction nX to mY, the value of DeltaH =-133 kJ and...
Text Solution
|
- For a hypothetical reaction nX to mY, the value of DeltaH =-133 kJ and...
Text Solution
|
- Compute the standard free energy of the reaction at 27^@C for the com...
Text Solution
|
- The reaction CH3COOH(l) + C2H5OH(l) hArr CH3 COOC2H5(l) + H2O (l) ...
Text Solution
|
- A system loses 120 ) of heat and does 80 J of work. Calculate the chan...
Text Solution
|
- Calculate the enthalpy change for the reaction CH2 = CH2(g) + H2(g0 ...
Text Solution
|
- Sort out the exothermic and endothermic reactions among the following:...
Text Solution
|
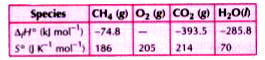
- Calculate the standard enthalpy change for the reaction CH4 (g) + ...
Text Solution
|
- Calculate the standard heat of formation of propane if its heat of com...
Text Solution
|
- When 14.9 g of solid KCl is dissolved in large excess of water, the am...
Text Solution
|
- what is entropy change for reaction 2H2(g) + O2 (g) -----?2H2O(l). Sta...
Text Solution
|
- Calculate the amount of heat liberated when 500 cm^3 " of " N/10 H2SO4...
Text Solution
|
- The standard molar heat of formation of ethane, carbon dioxide and wat...
Text Solution
|
- The Delta H^@ for the reaction H2(g) + I2(g) to 2HI(g) is +53.6 ...
Text Solution
|
- The heat of combustion of butane is 2658 kl. A cylinder of LPG gas (co...
Text Solution
|
- The calorific value of milk is 3.2 kJ g^(-1). A child needs about 4000...
Text Solution
|
- Sucrose undergoes combustion as C12 H22 O11 + 12 O2(g) to 12CO2(g)...
Text Solution
|
- Calculate the heat of formation of methanol (CH3OH) from thhe followin...
Text Solution
|
- Calculate the heat of formation of MgCO2 (s) from the following data ...
Text Solution
|
- Calculate the bond energy of H-Cl bond, given that the bond energies o...
Text Solution
|