A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL THERMODYNAMICS
ICSE|Exercise TRUE OR FALSE. TYPE QUESTIONS|24 VideosCHEMICAL THERMODYNAMICS
ICSE|Exercise FILL IN THE BLANKS. TYPE QUESTIONS|20 VideosCHEMICAL THERMODYNAMICS
ICSE|Exercise ESSAY (LONG ANSWER) TYPE QUESTIONS|32 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
ICSE|Exercise NCERT TEXT-BOOK EXERCISES ( WITH HINTS AND SOLUTIONS)|47 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
ICSE|Exercise NCERT TEXT-BOOK. EXERCISE (With Hints and Solutions)|63 Videos
Similar Questions
Explore conceptually related problems
ICSE-CHEMICAL THERMODYNAMICS -OBJECTIVE (MULTIPLE CHOCE) TYPE QUESTIONS
- A piston filled with 0.04 mole of an ideal gas expands reversibly from...
Text Solution
|
- For complete combustion of ethanol, C(2)H(5)OH(l) + 3O(2)(g) rarr 2CO(...
Text Solution
|
- A gas is allowed to expand in a well insulated container against a co...
Text Solution
|
- For a given reaction, DeltaH = 35.5 kJ mol^(-1) and Delta S = 83.6 JK...
Text Solution
|
- Given C(("graphite"))+O(2)(g)toCO(2)(g), Delta(r)H^(0)=-393.5kJ" "mo...
Text Solution
|
- DeltaU is equal to
Text Solution
|
- The bond dissociation energies of X2, Y2 and XY are in the ratio of 1 ...
Text Solution
|
- The combustion of benzene(/) gives CO2(g) and H2O(l) Given that heat o...
Text Solution
|
- For the cell reaction 2Fe^(3+)(aq) + 2l^(-1) (aq) to 2Fe^(2+) (aq) +...
Text Solution
|
- Under isothermal condition, a gas at 300 K expands from 0.1 L to 0.25 ...
Text Solution
|
- In which case change in entropy is negative?
Text Solution
|
- For a cell involving one electron E(cell)^(0)=0.59V and 298K, the equi...
Text Solution
|
- The INCORRECT match in the following is
Text Solution
|
- During compression of a spring the work done is 10 kJ and 2 kJ escaped...
Text Solution
|
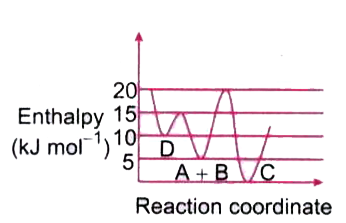
- Consider the given plot of enthalpy of the following reaction between ...
Text Solution
|
- The standard Gibbs energy for the given cell reaction in kJ mol^(-1) a...
Text Solution
|
- Among the following, the set of parameters that represents path functi...
Text Solution
|
- Which one of the following equations does not correctly represent the ...
Text Solution
|
- For silver, C(P)(J K^(-1)"mol"^(-1))=23+0.01T. If the temperature (T) ...
Text Solution
|
- 5 moles of an ideal gas at 100 K are allowed to undergo reversible com...
Text Solution
|