Text Solution
Verified by Experts
Topper's Solved these Questions
ANALYTICAL CHEMISTRY
ICSE|Exercise QUESTIONS FOR PRACTICE ON EXAMINATION PATTERN|20 VideosANALYTICAL CHEMISTRY
ICSE|Exercise Fill in the Blanks|17 VideosANALYTICAL CHEMISTRY
ICSE|Exercise Figure Based Questions|2 VideosACIDS, BASES AND SALTS
ICSE|Exercise Unit Test Paper 3A- Acids, Bases & Salts|14 VideosANALYTICAL CHEMISTRY- USE OF AMMONIUM HYDROXIDE AND SODIUM HYDROXIDE
ICSE|Exercise Questions from Previous ICSE Board Papers|45 Videos
Similar Questions
Explore conceptually related problems
ICSE-ANALYTICAL CHEMISTRY-QUESTIONS FOR PRACTICE
- You are provided with solutions of sodium hydroxide and ammonium hydro...
Text Solution
|
- You are provided with solutions of sodium hydroxide and ammonium hydro...
Text Solution
|
- solution will give a white precipitate with excess ammonium hydroxide ...
Text Solution
|
- You are provided with solutions of sodium hydroxide and ammonium hydro...
Text Solution
|
- You are provided with solutions of sodium hydroxide and ammonium hydro...
Text Solution
|
- You are provided with solutions of sodium hydroxide and ammonium hydro...
Text Solution
|
- Identify the cations in each of the following cases : (i) NaOH solutio...
Text Solution
|
- You are provided with solutions of sodium hydroxide and ammonium hydro...
Text Solution
|
- On reaction of sodium hydroxide with Iron(II) ion gives the coloured p...
Text Solution
|
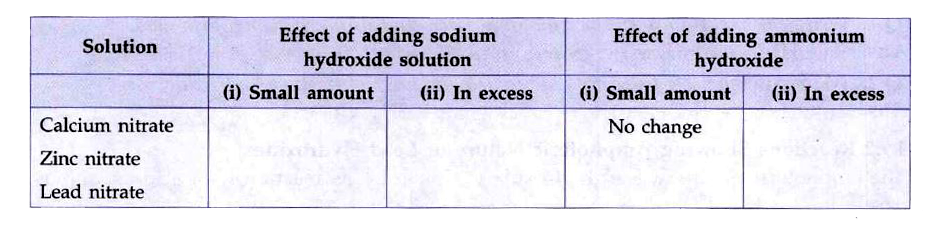
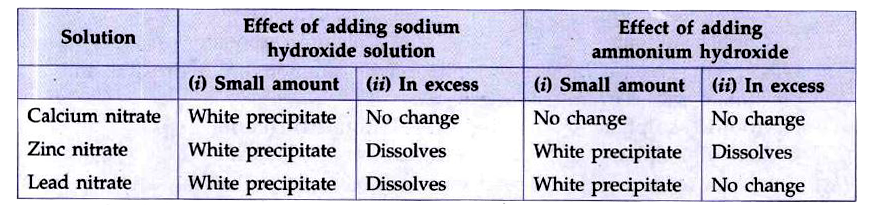
- Complete the following table which summarises the effect of adding a s...
Text Solution
|
- Give an example of a metallic oxide that forms salt and water with aci...
Text Solution
|
- Write the reactions of zinc oxide separately with HCl and NaOH.
Text Solution
|
- Write the reactions of aluminium oxide separately with HCl and NaOH.
Text Solution
|
- Excess of sodium hydroxide is added to zinc hydroxide.
Text Solution
|
- dilute hydrochloric acid is added to zinc hydroxide.
Text Solution
|
- Excess of sodium hydroxide is added to zinc hydroxide.
Text Solution
|
- dilute hydrochloric acid is added to zinc hydroxide.
Text Solution
|
- Identify the salts P from the observations given below: On perform...
Text Solution
|
- Identify the salts Q from the observations given below: When dilute...
Text Solution
|
- Select correct answers from the choices A, B, C, D which are given. Wr...
Text Solution
|