Topper's Solved these Questions
MOLE CONCEPT & STOICHIOMETRY (PART-A GAY LUSSAC'S LAW - AVOGADRO'S LAW-MOLE CONCEPT)
ICSE|Exercise Problems based on Mole Concept & Avogadro.s Number|13 VideosMOLE CONCEPT & STOICHIOMETRY (PART-A GAY LUSSAC'S LAW - AVOGADRO'S LAW-MOLE CONCEPT)
ICSE|Exercise Problems based on Mole Concept & Avogadro.s Law|2 VideosACIDS, BASES AND SALTS
ICSE|Exercise Unit Test Paper 3A- Acids, Bases & Salts|14 Videos
Similar Questions
Explore conceptually related problems
ICSE- MOLE CONCEPT & STOICHIOMETRY (PART-A GAY LUSSAC'S LAW - AVOGADRO'S LAW-MOLE CONCEPT)-Additional Problems (Vapour Density & Molecular Weight)
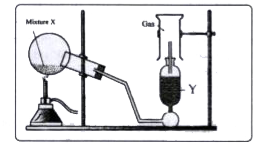
- The diagram shows an experimental set up for the laboratory preparatio...
Text Solution
|
- 500 ml. of a gas 'X' at s.t.p. weighs 0.50 g. Calculate the vapour den...
Text Solution
|
- A gas cylinder holds 85 g of a gas 'X'. The same cylinder when filled ...
Text Solution
|
- Calculate the relative molecular mass [molecular weight] of 290 ml of ...
Text Solution
|
- State the volume of occupied by 40 g. of a hydrocarbon - CH(4) at s.t....
Text Solution
|
- Calculate the atomicity of a gas X [at. No. 35.5] whose vapour density...
Text Solution
|
- Calculate the relative molecular mass vapour density of methyl alcohol...
Text Solution
|