Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-METALS AND NON-METALS-EXERCISE
- What is ductility?
Text Solution
|
- Explain the physical properties of metals with suitable examples.
Text Solution
|
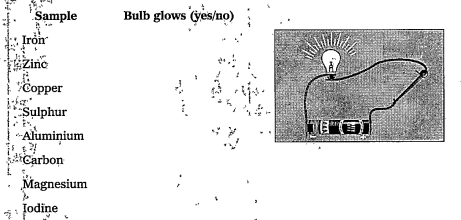
- Identifying the electric conductivity of a material: Arrange an elect...
Text Solution
|
- You are given two samples. How do you distinguish which one is metal a...
Text Solution
|
- Which metals are used in making jewellery?Why?
Text Solution
|
- Why cooking pans don't have metal handles?
Text Solution
|
- Dumping of waste material made up of metals and non metals leads to en...
Text Solution
|
- In a chemical reaction iron is unable to displace zinc from zinc sulph...
Text Solution
|
- How is malleability of metals used in our daily life?
Text Solution
|
- Sulphur dioxide is……..
Text Solution
|
- Maximum metals are obtained in the state of
Text Solution
|
- Which gas is liberated when, acids react with metals ? Give one exampl...
Text Solution
|
- Non-metallic oxides are innature
Text Solution
|
- Write an experiment to know the reaction of oxygen with metals and non...
Text Solution
|
- The nature of oxides helps to identify the metals and non metals condu...
Text Solution
|
- Identifying the electric conductivity of a material: Arrange an elect...
Text Solution
|
- How is malleability of metals used in our daily life?
Text Solution
|
- Imagine the human life without metals, write briefly about the consequ...
Text Solution
|
- Can you name some objects made of metals?
Text Solution
|
- Did any of your friends add steel to the list of metals?
Text Solution
|