Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SELF ASSESSMENT PAPER 1-SECTION-II
- What is meant by a group in the periodic table?
Text Solution
|
- Within a group where would you expect to find the element with (A) ...
Text Solution
|
- State whether the ionization potential decreases on going down a group...
Text Solution
|
- How many elements are there in period 2?
Text Solution
|
- Rewrite the following sentences by using the correct symbol gt (gre...
Text Solution
|
- The electronegativity of iodine is that of chlorine. (gt//lt )
Text Solution
|
- Give reasons why? Ionic compounds do not conduct electric current i...
Text Solution
|
- Give reasons why? Electrolysis is an example of a redox reaction.
Text Solution
|
- Give reasons why? For the preparation of hydrochloric acid, hydroge...
Text Solution
|
- Give reasons why? Tap water is not used to prepare a solution of s...
Text Solution
|
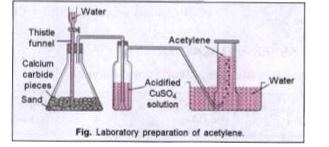
- Write the equation to explain the reaction taking place in the above d...
Text Solution
|
- Give the function of acidified copper sulphate solution.
Text Solution
|
- Give a reaction in which acetylene gas is prepared by synthesis reacti...
Text Solution
|
- (a) What is the reaction which a taking place ? (b) Give the functio...
Text Solution
|
- What happens when acetylene is heated in copper tube at 600^(@)C?
Text Solution
|
- Define the term "Catenation”.
Text Solution
|
- Conversion of ethene to ethane is an example of (hydration/hydrogenati...
Text Solution
|
- The catalyst used for conversion of ethene to ethane is commonly (nic...
Text Solution
|
- The product formed when ethene gas reacts with water in the presence o...
Text Solution
|
- burns with a non-luminous flame. (butane/methane/acetylene).
Text Solution
|