Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SELF ASSESSMENT PAPER 3 -SECTION-II
- The following questions related to the extraction of aluminium by elec...
Text Solution
|
- The following questions related to the extraction of aluminium by elec...
Text Solution
|
- The following questions related to the extraction of aluminium by elec...
Text Solution
|
- Arrange the following as per the instructions given in the brackets: ...
Text Solution
|
- Arrange the following as per the instructions given in the brackets :...
Text Solution
|
- Arrange the following as per the instructions given in the brackets :...
Text Solution
|
- How does ammonium hydroxide help in distinguish between: Iron (II) ...
Text Solution
|
- How does ammonium hydroxide help in distinguish between: Zinc nitrat...
Text Solution
|
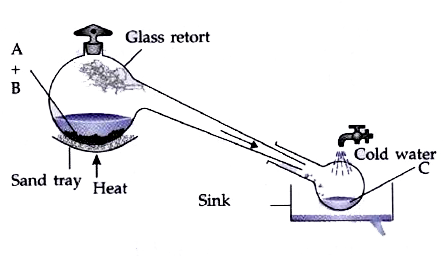
- The figure given alongside illustrates the apparatus used in the labor...
Text Solution
|
- Write an equation to show how nitric acid undergoes decomposition.
Text Solution
|
- The figure given below illustrates the apparatus used in the laborator...
Text Solution
|
- When moist chlorine reacts with hydrogen sulphide, name the two produc...
Text Solution
|
- Name an organic compound used as a thermometric liquid.
Text Solution
|
- Why is hydrogen chloride not collected over water ?
Text Solution
|
- Name the chemical in which gold can be dissolved.
Text Solution
|
- Name : a yellow monoxide that dissolves in hot and concentrated caus...
Text Solution
|
- Match the following columns
Text Solution
|
- Complete the following chemical equations : Al(4)C(3)+12H(2)O to
Text Solution
|
- Complete the following chemical equations : CaC(2)+2H(2)O overset(2...
Text Solution
|
- Complete the following chemical equations : C(2)H(5)Br overset(KOH)...
Text Solution
|